��Ŀ����
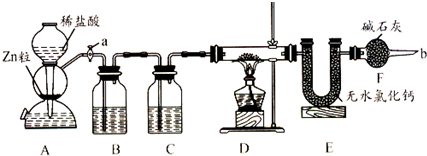
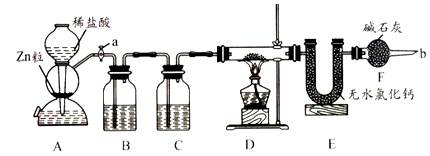
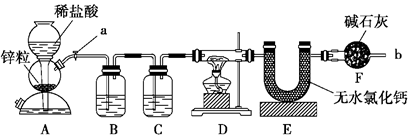
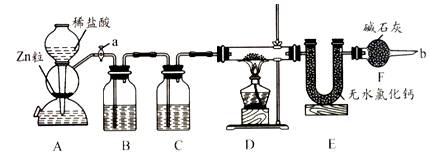
ʵ����������װ�òⶨFeO��Fe2O3����������Fe2O3��������Dװ�õ�Ӳ��˫ͨ�������еĹ���������FeO��Fe2O3�Ļ���

(1)��μ��װ��A��������________��
(2)Ϊ�˰�ȫ���ڵ�ȼD���ľƾ���֮ǰ����b������________��
(3)װ��B��������________��װ��C��װ��Һ����________��
(4)����������ã����ҽ����˱�Ҫ�İ�ȫ������ȼD���ľƾ��ƣ���Ӳ��˫ͨ�������з����Ļ�ѧ��Ӧ����ʽ��________��
(5)��FeO��Fe2O3�������������Ϊ23.2 g����Ӧ��ȫ��U�ܵ���������7.2 g��������Fe2O3������Ϊ________��
�𰸣�

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
�����Ŀ