��Ŀ����
(12��)
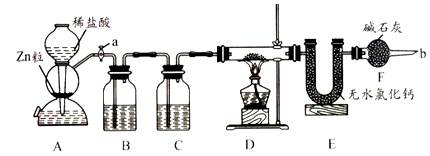
ʵ����������װ�òⶨFeO��Fe2O3����������Fe2O3��������Dװ�õ�Ӳ��˫ͨ�������еĹ���������FeO��Fe2O3�Ļ���
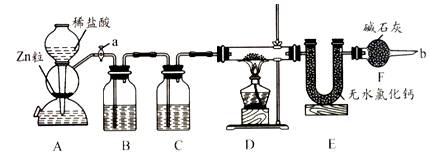
��1����μ��װ��A��������
��2��Ϊ�˰�ȫ���ڵ�ȼD���ľƾ���֮ǰ����b������ ��
(3)װ��B��������
װ��C��װ��Һ����
��4������������ã����ҽ����˱�Ҫ�İ�ȫ������ȼD���ľƾ��ƣ���Ӳ��˫ͨ�������з����Ļ�ѧ��Ӧ����ʽ��
��5����FeO��Fe2O3�������������Ϊ23. 2g����Ӧ��ȫ��U�ܵ���������7.2g��������Fe2O3������Ϊ _��
��1���֣�
��1��������������©���м�ˮ����ˮ���������²��İ�����ʱ���ر�������������ˮ��ʹˮ����������©���С�����Ƭ�̣���ˮ�治�½�����˵��װ������������
��2���鴿
��3����HCl��ŨH2SO4
��4��Fe2O3+3H2=2Fe+3H2O FeO+H2=Fe+H2O
��5��16g
���������������������ȡ�����ʵIJⶨ�����ʵ�����
��3��װ��B��CΪ����ľ���װ�ã��ֱ��ȥ�������HCl��ˮ������
��4�����ݻ���������������ˮ�������г���������㼴�ɡ�

 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
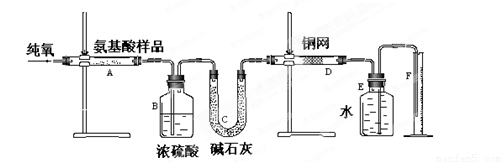
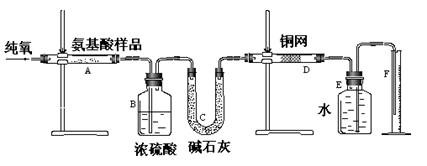
����˼ά����ѵ����ʱ��ѧ��ϵ�д�(12��)ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣�ش��������⣺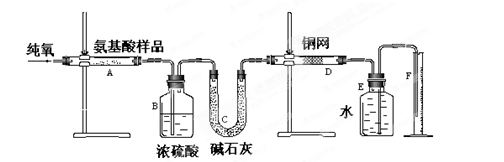
(1)ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������_ ��
(2)����װ������Ҫ���ȵ�������______(��д��ĸ)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________ ___________________��
(4)Dװ�õ������� ��
(5)ʵ���в�õ��������ΪV mL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������_ __��
| A�����ɶ�����̼��������� | B������ˮ������ |
| C��ͨ����������� | D�����������Է������� |