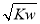جâؤ؟ؤعبف
ؤ³¶شھثل(H2A)°´دآت½·¢ةْµçہë£؛H2A=H£«£«HA££»HA£ H£«£«A2£،£دضسذدآءذثؤضضبـز؛£؛
H£«£«A2£،£دضسذدآءذثؤضضبـز؛£؛
¢ظ0.01 mol،¤L£1µؤH2Aبـز؛
¢ع0.01 mol،¤L£1µؤNaHAبـز؛
¢غ0.02 mol،¤L£1µؤHClبـز؛سë0.04 mol،¤L£1µؤNaHAبـز؛µبجه»»ى؛د
¢ـ0.02 mol،¤L£1µؤNaOHبـز؛سë0.02 mol،¤L£1µؤNaHAبـز؛µبجه»»ى؛د
دآءذ¹طسعةدتِثؤضضبـز؛µؤثµ·¨²»صب·µؤتا(،،،،)
A£®بـز؛¢عضذ´وشعث®½âئ½؛â£؛HA££«H2O H2A£«OH£
H2A£«OH£
B£®بـز؛¢غضذسذ£؛c(HA£)£«c(A2£)£½c(Na£«)
C£®بـز؛¢ـضذسذ£؛c(OH£)£½c(H£«)£«c(HA£)
D£®ثؤضضبـز؛ضذc(HA£)´َذ،£؛¢غ>¢ظ>¢ع>¢ـ
A
،¾½âخِ،؟زٍخھH2Aحêب«µçہë³ةHA££¬ثùزشHA£²»ؤـث®½â£¬A´يخَ،£سةخïءدتط؛م؟ةضھبـز؛¢غضذسذ£؛c(HA£)£«c(A2£)£½c(Na£«)£¬شٍBصب·،£سةضت×ستط؛م؟ةضھبـز؛¢ـضذسذ£؛c(OH£)£½c(H£«)£«c(HA£)£¬Cصب·،£¢ظ،¢¢عضذc(HA£)<0.01 mol،¤L£1£¬µ«¢ظضذH£«µؤ´وشعزضضئHA£µؤµçہ룬¹تc(HA£)£؛¢ظ>¢ع،¢¢غضذثلµؤ´وشعزضضئHA£µؤµçہ룬c(HA£)آشذ،سع0.02 mol،¤L£1£»¢ـضذء½خïضتا،؛أحêب«·´س¦ةْ³ةNa2A£¬A2£ث®½âةْ³ةµؤHA£تا¼«ةظµؤ،£