��Ŀ����
ij��ȤС����Ʋ�����������ʵ����̽��Cl2��Ư�۵��Ʊ����й����ʡ�
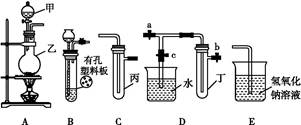
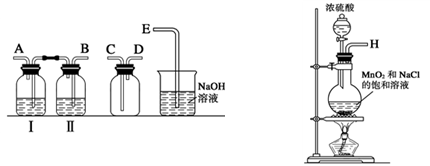
��1��ʵ������������װ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�
H�������������������������������������������������������ƿ���е��Լ�Ϊ��������������������������
��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
������������������������������������������������������������������������������������������
ijѧ���������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ��
��3��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������������������������������������������
��4����ͬѧ�Ʋ�����i����״��������СҺ���γɣ���������ʵ����Խ�һ����֤��
a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯��
b���Ѽ���״����ữ��AgNO3��Һ���飬������ɫ������
ʵ��a��Ŀ��������������������������������������������������������������������������
��5����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾���ijЩ�ɷ�֮�䷢����Ӧ�������ʵ�鷽������һ��ȷ�����ֿ����ԣ�����Ϊ��������������������������������������������
������������������������������������������������������������������������������������
��6���û�ѧ����ʽ���������л���ɫ��ȥ��ԭ����������������������ɫ����Һ���Ƿ���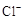 �ķ����ǣ��� ����������������������������������������
�ķ����ǣ��� ����������������������������������������
��1��ʵ������������װ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�
H�������������������������������������������������������ƿ���е��Լ�Ϊ��������������������������

��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
������������������������������������������������������������������������������������������
ijѧ���������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ��
| ���� | ���� |
| ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
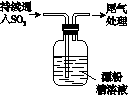 A | ��.Һ���Ϸ�������״�� ��.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ ��.�Ժ���������ɫ����������ɫ��ȥ |
��3��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������������������������������������������
��4����ͬѧ�Ʋ�����i����״��������СҺ���γɣ���������ʵ����Խ�һ����֤��
a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯��
b���Ѽ���״����ữ��AgNO3��Һ���飬������ɫ������
ʵ��a��Ŀ��������������������������������������������������������������������������
��5����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾���ijЩ�ɷ�֮�䷢����Ӧ�������ʵ�鷽������һ��ȷ�����ֿ����ԣ�����Ϊ��������������������������������������������
������������������������������������������������������������������������������������
��6���û�ѧ����ʽ���������л���ɫ��ȥ��ԭ����������������������ɫ����Һ���Ƿ���
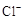 �ķ����ǣ��� ����������������������������������������
�ķ����ǣ��� ������������������������������������������1��BACDE (1��)������ʳ��ˮ(1��)
��2��2Ca(OH)2 +2 Cl2 ��Ca(ClO)2 +CaCl2 +2H2O (2��)
��3�����ԡ�Ư���� (2��)
��4���ų���������(1�֣���������)
��5����Ư�۾���Һ����μ������ᣬ�۲���Һ�Ƿ��Ϊ����ɫ(1�֣���������)
��6��SO2 +Cl2 +2H2O �� H2SO4 +2HCl (2��)��ȡ�����������������ᱵ��������������ȡ�ϲ���ҹ��������������ϡ���ᣬ������ɫ������˵����Cl����(2��)
��2��2Ca(OH)2 +2 Cl2 ��Ca(ClO)2 +CaCl2 +2H2O (2��)
��3�����ԡ�Ư���� (2��)
��4���ų���������(1�֣���������)
��5����Ư�۾���Һ����μ������ᣬ�۲���Һ�Ƿ��Ϊ����ɫ(1�֣���������)
��6��SO2 +Cl2 +2H2O �� H2SO4 +2HCl (2��)��ȡ�����������������ᱵ��������������ȡ�ϲ���ҹ��������������ϡ���ᣬ������ɫ������˵����Cl����(2��)
�����������1���Ʊ�������ʵ���У�Ҫ��ȥ�����Ȼ����ˮ�����������õ�ϴ��װ�ã�һ���dz����̳������˳��Ϊ��B A C D E������Ϊ���ȳ�ȥ������е��Ȼ��⣬��˹��ƿ���е��Լ�Ϊ����ʳ��ˮ���������ڱ���ʳ��ˮ���ܽ�Ƚ�С����
��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��2Ca(OH)2 +2 Cl2 ��Ca(ClO)2 +CaCl2 +2H2O ��
��3��pH��ֽ��ɫ�ȱ���˵���ʼ��ԣ�����ɫ˵��Ư�۾���Ư���ԡ�
��4����ͬѧ�Ʋ�����i����״��������СҺ���γɣ���������ʵ����Խ�һ����֤��a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯Ŀ�����ų��������š�
��5����������Һ��Ϊ����ɫ˵�����������ɣ�ʵ���Ǵ����������������������Һ�з����˷�Ӧ��ֻҪ���ò��������ӵ���Һ���Խ�����֤���ɣ�����Ϊ��Ư�۾���Һ����μ������ᣬ�۲���Һ�Ƿ��Ϊ����ɫ��
��6�����л���ɫ��ȥ��ԭ�Ƕ���������л�ԭ�Ժ���������������ԭ��Ӧ��SO2 +Cl2 +2H2O �� H2SO4 +2HCl��������ɫ����Һ���Ƿ��������ӵķ�����ȡ�����������������ᱵ��������������ȡ�ϲ���ҹ��������������ϡ���ᣬ������ɫ������˵����Cl����2��Ư�۵��Ʊ����й����ʡ�

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ