��Ŀ����
2011��9��25�յ�26�������������������人��Ļ���й��ӻ�ùھ���ȡ���ذ��˻��볡ȯ�����������в���ȷ���ǣ�������
������A�����ݸֽ�������ijɷֺ����ʷ����жϣ�
B�����������е�������ˮ����������������������жϣ�
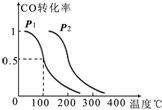
C������̫���ܵ�ؿ�ʹ����ת��Ϊ���ܣ������в������������ʴﵽ100%��
D����������β���е���Ⱦ�����ڴ������������������������
B�����������е�������ˮ����������������������жϣ�
C������̫���ܵ�ؿ�ʹ����ת��Ϊ���ܣ������в������������ʴﵽ100%��
D����������β���е���Ⱦ�����ڴ������������������������
����⣺A���ֽ�����������㷺Ӧ���ڽ����ṹ�У�����������֮ǰ���Ƚ��а��֧ģ��Ҳ��������˿���ֽ�̶�����Ҫ�Ľṹ��״��Ȼ����ģ�帲���ڸֽ�Ǽ����森�������������ȥ�������ڸ��ϲ��ϣ���A��ȷ��
B������ˮʱ�������������������ӣ���ˮ��Һ��ˮ�����������������壬�����������ã���Ϊ��ˮ��������ˮ�е����ʣ���B��ȷ��
C��̫���ܵ�ز���ʹ���������ʴﵽ100%����ת�������л�����������ʽת�������ģ���C����
D����װ���ⷴӦ��ʩ���ɽ�����β����CO��NOx��Ӧ���������嵪���Ͷ�����̼������������������Ⱦ�����ã���D��ȷ��
��ѡC��
B������ˮʱ�������������������ӣ���ˮ��Һ��ˮ�����������������壬�����������ã���Ϊ��ˮ��������ˮ�е����ʣ���B��ȷ��
C��̫���ܵ�ز���ʹ���������ʴﵽ100%����ת�������л�����������ʽת�������ģ���C����
D����װ���ⷴӦ��ʩ���ɽ�����β����CO��NOx��Ӧ���������嵪���Ͷ�����̼������������������Ⱦ�����ã���D��ȷ��
��ѡC��
���������⿼���˲��ϵijɷֺͷ��࣬����ˮ���Ӧ�ã�����β���Ĺ��ת�����ã��ϼ�

��ϰ��ϵ�д�
�����Ŀ