��Ŀ����
ʵ�֡����ܼ��š��͡���̼���á���һ����Ҫ������� ��ν�CO2ת��Ϊ�����õ���Դ��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��
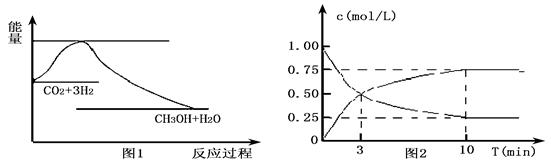
CO2��g��+3H2��g���TCH3OH��g��+H2O��g����ͼ1��ʾ�÷�Ӧ��������������λΪkJ?mol-1���ı仯��
��1�����ڸ÷�Ӧ������˵���У���ȷ����
A����H��0����S��0 B����H��0����S��0 C����H��0����S��0 D����H��0����S��0
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊl L���ܱ������У�����l mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g�������CO2��CH3OH��g����Ũ����ʱ��仯����ͼ2��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ����v��CH3OH��=
�ڸ÷�Ӧ��ƽ�ⳣ������ʽK=
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����
A�������¶� B����CH3OH��g����ʱҺ�����
C��ѡ���Ч���� D���ٳ���l molCO2��3molH2
��3��25�棬1.01��105Paʱ��16g Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ��������д����ʾCH3OHȼ���ȵ��Ȼ�ѧ����ʽ��

CO2��g��+3H2��g���TCH3OH��g��+H2O��g����ͼ1��ʾ�÷�Ӧ��������������λΪkJ?mol-1���ı仯��
��1�����ڸ÷�Ӧ������˵���У���ȷ����
C
C
������ĸ����A����H��0����S��0 B����H��0����S��0 C����H��0����S��0 D����H��0����S��0
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊl L���ܱ������У�����l mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g�������CO2��CH3OH��g����Ũ����ʱ��仯����ͼ2��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ����v��CH3OH��=
0.075mol
0.075mol
mol?��L?min��-1��H2��ת����=75%
75%
�ڸ÷�Ӧ��ƽ�ⳣ������ʽK=
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����
BD
BD
������ĸ����A�������¶� B����CH3OH��g����ʱҺ�����
C��ѡ���Ч���� D���ٳ���l molCO2��3molH2
��3��25�棬1.01��105Paʱ��16g Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ��������д����ʾCH3OHȼ���ȵ��Ȼ�ѧ����ʽ��
CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-726.6 kJ?mol-1
| 3 |
| 2 |
CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-726.6 kJ?mol-1
��| 3 |
| 2 |
��������1�����ݷ�Ӧ��ͼ������жϣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g������������ʵ������٣���Ӧ���ؼ��ٵķ�Ӧ��S��0����Ӧ������������������������жϷ�Ӧ�Ƿ��ȷ�Ӧ����H��0��
��2��������̼�Ƿ�Ӧ���淴Ӧ����Ũ�ȼ�С���״���������淴Ӧ����Ũ������10nim�ڴﵽƽ�⣬���ɼ״�Ũ��Ϊ0.75mol/L��������̼Ũ�ȱ仯��0.75mol/L��
�ٸ���c=
����v��CH3OH����
����Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȼ����c��H2��������μӷ�Ӧ�����������ʵ���������ת���ʵ��ڼ���������ת���ʣ�
��ƽ�ⳣ�������������Ũ�Ȼ�ѧ��������֮�����Է�Ӧ���Ũ�Ȼ�ѧ��������֮����
�����ݻ�ѧ��Ӧ��Ӱ�����غ�������������жϣ�
A����Ӧ�Ƿ��ȷ�Ӧ������ƽ��������У�
B����CH3OH��g����ʱҺ���������С���������ƽ��������У�
C��ѡ���Ч����ֻ�ܸı����ʣ����ı仯ѧƽ�⣻
D���ٳ���l molCO2��3molH2������ѹǿƽ��������У�
��3��25�棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ����������1mol�״���ȫȼ�ջָ���ԭ״̬ʱ���ų�����=363.3kJ��
=726.6kJ��������д�Ȼ�ѧ����ʽ�ķ���д���״�ȼ���ȵ��Ȼ�ѧ����ʽ��
��2��������̼�Ƿ�Ӧ���淴Ӧ����Ũ�ȼ�С���״���������淴Ӧ����Ũ������10nim�ڴﵽƽ�⣬���ɼ״�Ũ��Ϊ0.75mol/L��������̼Ũ�ȱ仯��0.75mol/L��
�ٸ���c=
| ��c |
| ��t |
����Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȼ����c��H2��������μӷ�Ӧ�����������ʵ���������ת���ʵ��ڼ���������ת���ʣ�
��ƽ�ⳣ�������������Ũ�Ȼ�ѧ��������֮�����Է�Ӧ���Ũ�Ȼ�ѧ��������֮����
�����ݻ�ѧ��Ӧ��Ӱ�����غ�������������жϣ�
A����Ӧ�Ƿ��ȷ�Ӧ������ƽ��������У�
B����CH3OH��g����ʱҺ���������С���������ƽ��������У�
C��ѡ���Ч����ֻ�ܸı����ʣ����ı仯ѧƽ�⣻
D���ٳ���l molCO2��3molH2������ѹǿƽ��������У�
��3��25�棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ����������1mol�״���ȫȼ�ջָ���ԭ״̬ʱ���ų�����=363.3kJ��
| 1mol��32g/mol |
| 16g |
����⣺��1�����ݷ�Ӧ��ͼ������жϣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g������������ʵ������٣���Ӧ���ؼ��ٵķ�Ӧ��S��0����Ӧ������������������������жϷ�Ӧ�Ƿ��ȷ�Ӧ����H��0��
�ʴ�Ϊ��C��
��2��������̼�Ƿ�Ӧ���淴Ӧ����Ũ�ȼ�С���״���������淴Ӧ����Ũ������10nim�ڴﵽƽ�⣬���ɼ״�Ũ��Ϊ0.75mol/L��������̼Ũ�ȱ仯��0.75mol/L����
��v��CH3OH��=
=0.075mol/��L?min����
Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ���c��H2��=0.75mol/L��3=2.25mol/L���μӷ�Ӧ�����������ʵ���=2.25mol/L��1L=2.25mol����������ת����=
��100%=75%��
�ʴ�Ϊ��0.075mol/��L?min����75%��
��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ƽ�ⳣ������ʽK=
��
�ʴ�Ϊ��
��
�۴�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ����ǣ�
A����Ӧ�Ƿ��ȷ�Ӧ������ƽ��������У���A����
B����CH3OH��g����ʱҺ���������С�����������ƽ��������У���B��ȷ��
C��ѡ���Ч����ֻ�ܸı����ʣ����ı仯ѧƽ�⣬��C����
D���ٳ���l molCO2��4molH2������ѹǿƽ��������У���D��ȷ��
��ѡ��BD��
��3��25�棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ����������1mol�״���ȫȼ�ջָ���ԭ״̬ʱ���ų�����=363.3kJ��
=726.6kJ���ʼ״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-726.6 kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-726.6 kJ?mol-1��
�ʴ�Ϊ��C��
��2��������̼�Ƿ�Ӧ���淴Ӧ����Ũ�ȼ�С���״���������淴Ӧ����Ũ������10nim�ڴﵽƽ�⣬���ɼ״�Ũ��Ϊ0.75mol/L��������̼Ũ�ȱ仯��0.75mol/L����
��v��CH3OH��=
| 0.75mol/L |
| 10min |
Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ���c��H2��=0.75mol/L��3=2.25mol/L���μӷ�Ӧ�����������ʵ���=2.25mol/L��1L=2.25mol����������ת����=
| 2.25mol |
| 3mol |
�ʴ�Ϊ��0.075mol/��L?min����75%��
��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ƽ�ⳣ������ʽK=
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
�ʴ�Ϊ��
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
�۴�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ����ǣ�
A����Ӧ�Ƿ��ȷ�Ӧ������ƽ��������У���A����
B����CH3OH��g����ʱҺ���������С�����������ƽ��������У���B��ȷ��
C��ѡ���Ч����ֻ�ܸı����ʣ����ı仯ѧƽ�⣬��C����
D���ٳ���l molCO2��4molH2������ѹǿƽ��������У���D��ȷ��
��ѡ��BD��
��3��25�棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ����������1mol�״���ȫȼ�ջָ���ԭ״̬ʱ���ų�����=363.3kJ��
| 1mol��32g/mol |
| 16g |
| 3 |
| 2 |
�ʴ�Ϊ��CH3OH��l��+
| 3 |
| 2 |
���������⿼���˷�Ӧ���ʱ��жϣ��Ȼ�ѧ����ʽ����д����ѧƽ���Ӱ�����أ���ѧƽ��ļ��㡢��ѧƽ�ⳣ���ȣ�ͼ������ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ

 CO2(g)+3H2(g)
CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��
CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��