��Ŀ����
�������洦����Ƥ�����ơ�ӡȾ�ȶ�������ɸ���Ⱦ�����۸������۸����Ըߣ����ױ����������������������
�Ź�ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ�� ��
�ڵ�����Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ ��25�棬��������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
�Ƹ�Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
�Ź�ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ�� ��
�ڵ�����Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ ��25�棬��������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
�Ƹ�Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
��Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O
�ڽ�pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ�� ����pH��ֽ�ϣ��������ɫ������
6.3��10��13
���ɷ���ʽ��֪��Cr~3Na2S2O3
n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol
n(Cr)=1��10��4mol
m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg
��ˮ�и�Ԫ����Ũ��=
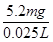 =208 mg��L��1
=208 mg��L��1�����������1���ٷ���������ԭ��Ӧ���������ӱ�����Ϊ�����ӣ�Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O����ʵ���Ҵ��Բⶨ��ҺpH�ķ�����ʹ��pH��ֽ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�����Һ�в���Cr3�������ʵ���Ũ��Ϊ
Ksp[Cr(OH)3]��(OH��)3=6.3��10��31��10��18=6.3��10��13��
��2���ɷ���ʽȷ����ϵʽ��Cr~3Na2S2O3
n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol
n(Cr)=1��10��4mol
m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg
��ˮ�и�Ԫ����Ũ��=
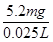 =208 mg��L��1
=208 mg��L��1
��ϰ��ϵ�д�
�����Ŀ
 �к����ӡ����ӡ�������Ŀ��ΪNA
�к����ӡ����ӡ�������Ŀ��ΪNA