��Ŀ����
 ��1����7.8�˹�������Ͷ�뵽100��10%�Ŀ�������Һ�г�ַ�Ӧ����Ӧ�����ӷ���ʽΪ��
��1����7.8�˹�������Ͷ�뵽100��10%�Ŀ�������Һ�г�ַ�Ӧ����Ӧ�����ӷ���ʽΪ����2��ʹ4.48L CO2����Ѹ��ͨ��Na2O2�����õ�3.36L����״���£����壬��3.36L�����������
��3����100mL 3mol/L AlCl3��Һ�еμ�1mol/L��NaOH��Һ����7.8g�����������NaOH��Һ�����������
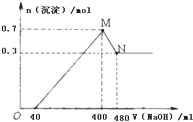
��4����һ��������Mg��Al�����Ͷ��400mLϡ�����У�����ȫ���ܽⲢ�������壮����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ�����㣺Mg��Al��������Ϊ
���㣺��ѧ����ʽ���йؼ���,�йػ���ﷴӦ�ļ���
ר�⣺������
��������1��������Ӧ2Na2O2+2H2O=4NaOH+O2�������ݷ���ʽ���㷴Ӧ���ɵ�NaOH������������������������ԭ��Һ��NaOH���������������㷴Ӧ����Һ��NaOH������������Ӧ����Һ������=������������+ԭ��Һ����-�����������ٸ�����������������㣻
��2���������������ͨ������������μӷ�Ӧ�Ķ�����̼��������ɵ�������������ж�4.48L�������ɼ����ɷֵ�������ٸ���n=
����������������ʵ���������m=nM����������
��3��100mL 3mol/L��AlCl3��Һ���Ȼ��������ʵ���=0.1L��3mol/L=0.3mol����AlԪ�ض�ת��Ϊ����������������������������������=0.3mol��78g/mol��7.8g��˵�������������һΪ��������ȫ��ֻ����Al��OH��3��������һ�����Ϊ���������ܽ⣬������Al��OH��3������������NaAlO2����Ϸ���ʽ�������������������ʵ���������������Ҫ����������Һ�����
��4����ͼ���֪���ӿ�ʼ������NaOH��Һ40mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH=Na2SO4+2H2O����V��NaOH��Һ��=400mLʱ�����������ʱΪMg��OH��2��Al��OH��3���������ʵ���֮��Ϊ0.7mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��Na2SO4������400mL����������Һ�к��е�n��NaOH����0.5������400mL��ʼ��NaOH�ܽ�Al��OH��3��������ӦNaOH+Al��OH��3=NaAlO2+2H2O�����������ټ��٣���ʱȫ��ΪMg��OH��2�����ʵ���Ϊ0.3mol�����Գ��������ʱ��Mg��OH��2Ϊ0.3mol��Al��OH��3Ϊ0.7mol-0.3mol=0.4mol�����Ըý�����n��NaOH��=n[Al��OH��3]=0.4mol���������Ƶ�Ũ��Ϊ
=5mol/L��
��Ԫ���غ��֪n��Al��=n[Al��OH��3]��n��Mg��=n[Mg��OH��2]���ڸ���m=nM������Ե����������������������������
���������ʱΪMg��OH��2��Al��OH��3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��NaOH��=2n��Na2SO4��������������غ�n��H2SO4��=n��Na2SO4�����ٸ���c=
���㣻
���ݵ���ת���غ��֪2n��H2��=3n��Al��+2n��Mg�����ݴ˼���n��H2����
��2���������������ͨ������������μӷ�Ӧ�Ķ�����̼��������ɵ�������������ж�4.48L�������ɼ����ɷֵ�������ٸ���n=
| V |
| Vm |
��3��100mL 3mol/L��AlCl3��Һ���Ȼ��������ʵ���=0.1L��3mol/L=0.3mol����AlԪ�ض�ת��Ϊ����������������������������������=0.3mol��78g/mol��7.8g��˵�������������һΪ��������ȫ��ֻ����Al��OH��3��������һ�����Ϊ���������ܽ⣬������Al��OH��3������������NaAlO2����Ϸ���ʽ�������������������ʵ���������������Ҫ����������Һ�����
��4����ͼ���֪���ӿ�ʼ������NaOH��Һ40mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH=Na2SO4+2H2O����V��NaOH��Һ��=400mLʱ�����������ʱΪMg��OH��2��Al��OH��3���������ʵ���֮��Ϊ0.7mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��Na2SO4������400mL����������Һ�к��е�n��NaOH����0.5������400mL��ʼ��NaOH�ܽ�Al��OH��3��������ӦNaOH+Al��OH��3=NaAlO2+2H2O�����������ټ��٣���ʱȫ��ΪMg��OH��2�����ʵ���Ϊ0.3mol�����Գ��������ʱ��Mg��OH��2Ϊ0.3mol��Al��OH��3Ϊ0.7mol-0.3mol=0.4mol�����Ըý�����n��NaOH��=n[Al��OH��3]=0.4mol���������Ƶ�Ũ��Ϊ
| 0.4mol/L |
| 0.48L-0.4L |
��Ԫ���غ��֪n��Al��=n[Al��OH��3]��n��Mg��=n[Mg��OH��2]���ڸ���m=nM������Ե����������������������������
���������ʱΪMg��OH��2��Al��OH��3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��NaOH��=2n��Na2SO4��������������غ�n��H2SO4��=n��Na2SO4�����ٸ���c=
| n |
| V |
���ݵ���ת���غ��֪2n��H2��=3n��Al��+2n��Mg�����ݴ˼���n��H2����
���
�⣺��1�������������Ƿ�Ӧ���ӷ���ʽΪ��2Na2O2+2H2O=4Na++4OH-+O2����
��7.8g�������Ʒ�Ӧ������������Ϊmg����������Ϊng����
2Na2O2+2H2O=4NaOH+O2����
2��78 160 32
7.8g mg ng
��2��78��160=7.8g��mg ���m=8��
2��78��32=7.8g��ng ���n=1.6��
ԭ��Һ��NaOH�����ʵ���=100g��100%=10g
�ʷ�Ӧ��NaOH��Һ����������=
��100%=16.9%��
�ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH-+O2����16.9%��
��2����μӷ�Ӧ��CO2��������Ϊa�����ɵ�O2�����Ϊb����
2CO2+2Na2O2=2Na2CO3+O2�������������V
2 1 1
a b 4.48L-3.36L=1.12L
���a=2.24L��b=1.12L
����3.36L������CO2���Ϊ3.36L-1.12L=2.24L��O2�����Ϊ1.12L��
����3.36L���������Ϊ
��44g/mol+
��32g/mol=6g��
�ʴ�Ϊ��6��
��3��100mL 3mol/L��AlCl3��Һ���Ȼ��������ʵ���=0.1L��3mol/L=0.3mol����AlԪ�ض�ת��Ϊ����������������������������������=0.3mol��78g/mol��7.8g��˵�������������һΪ��������ȫ��ֻ����Al��OH��3��������һ�����Ϊ���������ܽ⣬������Al��OH��3������������NaAlO2��
n��Al��OH��3��=
=0.1mol��
������㣬��Al3++3OH-�TAl��OH��3����֪��NaOH�����ʵ���Ϊ0.1mol��3=0.3mol��
����NaOH��Һ�����Ϊ
=0.3L��
�ڳ��������ܽ⣬������Al��OH��3������������NaAlO2����
Al3++3OH-�TAl��OH��3��
0.3mol 0.9mol 0.3mol
Al��OH��3+OH-�TAlO2-+2H2O
��0.3-0.1��mol ��0.3-0.1��mol
�����ĵļ�����ʵ���Ϊ0.9mol+��0.3-0.1��mol=1.1mol��
����NaOH��Һ�����Ϊ
=1.1L��
�ʴ�Ϊ��0.3L��1.1L��
��4����ͼ���֪���ӿ�ʼ������NaOH��Һ40mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH=Na2SO4+2H2O����V��NaOH��Һ��=400mLʱ�����������ʱΪMg��OH��2��Al��OH��3���������ʵ���֮��Ϊ0.7mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��Na2SO4������400mL����������Һ�к��е�n��NaOH����0.5������400mL��ʼ��NaOH�ܽ�Al��OH��3��������ӦNaOH+Al��OH��3=NaAlO2+2H2O�����������ټ��٣���ʱȫ��ΪMg��OH��2�����ʵ���Ϊ0.3mol�����Գ��������ʱ��Mg��OH��2Ϊ0.3mol��Al��OH��3Ϊ0.7mol-0.3mol=0.4mol�����Ըý�����n��NaOH��=n[Al��OH��3]=0.4mol���������Ƶ�Ũ��Ϊ
=5mol/L��
��Ԫ���غ��֪n��Al��=n[Al��OH��3]=0.4mol��n��Mg��=n[Mg��OH��2]=0.3mol����Mg��Al��������Ϊ0.4mol��27g/mol+0.3mol��24g/mol=18g��
���������ʱΪMg��OH��2��Al��OH��3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��NaOH��=2n��Na2SO4��=0.4L��5mol/L=2mol������n��Na2SO4��=1mol�����������Ũ��Ϊ
=2.5mol/L��
�ɣ�1���п�֪n��Al��=0.4mol��n��Mg��=0.3mol�����ݵ���ת���غ��֪2n��H2��=3n��Al��+2n��Mg��=3��0.4mol+2��0.3mol=1.8mol������n��H2��=0.9mol��
�ʴ�Ϊ��18g��2.5mol/L��0.9mol��
��7.8g�������Ʒ�Ӧ������������Ϊmg����������Ϊng����
2Na2O2+2H2O=4NaOH+O2����
2��78 160 32
7.8g mg ng
��2��78��160=7.8g��mg ���m=8��
2��78��32=7.8g��ng ���n=1.6��
ԭ��Һ��NaOH�����ʵ���=100g��100%=10g
�ʷ�Ӧ��NaOH��Һ����������=
| 8g+10g |
| 7.8g+100g-1.6g |
�ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH-+O2����16.9%��
��2����μӷ�Ӧ��CO2��������Ϊa�����ɵ�O2�����Ϊb����
2CO2+2Na2O2=2Na2CO3+O2�������������V
2 1 1
a b 4.48L-3.36L=1.12L
���a=2.24L��b=1.12L
����3.36L������CO2���Ϊ3.36L-1.12L=2.24L��O2�����Ϊ1.12L��
����3.36L���������Ϊ
| 2.24L |
| 22.4L/mol |
| 1.12L |
| 22.4L/mol |
�ʴ�Ϊ��6��
��3��100mL 3mol/L��AlCl3��Һ���Ȼ��������ʵ���=0.1L��3mol/L=0.3mol����AlԪ�ض�ת��Ϊ����������������������������������=0.3mol��78g/mol��7.8g��˵�������������һΪ��������ȫ��ֻ����Al��OH��3��������һ�����Ϊ���������ܽ⣬������Al��OH��3������������NaAlO2��
n��Al��OH��3��=
| 7.8g |
| 78g/mol |
������㣬��Al3++3OH-�TAl��OH��3����֪��NaOH�����ʵ���Ϊ0.1mol��3=0.3mol��
����NaOH��Һ�����Ϊ
| 0.3mol |
| 1mol/L |
�ڳ��������ܽ⣬������Al��OH��3������������NaAlO2����
Al3++3OH-�TAl��OH��3��
0.3mol 0.9mol 0.3mol
Al��OH��3+OH-�TAlO2-+2H2O
��0.3-0.1��mol ��0.3-0.1��mol
�����ĵļ�����ʵ���Ϊ0.9mol+��0.3-0.1��mol=1.1mol��
����NaOH��Һ�����Ϊ
| 1.1mol |
| 1mol/L |
�ʴ�Ϊ��0.3L��1.1L��
��4����ͼ���֪���ӿ�ʼ������NaOH��Һ40mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH=Na2SO4+2H2O����V��NaOH��Һ��=400mLʱ�����������ʱΪMg��OH��2��Al��OH��3���������ʵ���֮��Ϊ0.7mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��Na2SO4������400mL����������Һ�к��е�n��NaOH����0.5������400mL��ʼ��NaOH�ܽ�Al��OH��3��������ӦNaOH+Al��OH��3=NaAlO2+2H2O�����������ټ��٣���ʱȫ��ΪMg��OH��2�����ʵ���Ϊ0.3mol�����Գ��������ʱ��Mg��OH��2Ϊ0.3mol��Al��OH��3Ϊ0.7mol-0.3mol=0.4mol�����Ըý�����n��NaOH��=n[Al��OH��3]=0.4mol���������Ƶ�Ũ��Ϊ
| 0.4mol |
| 0.48L-0.4L |
��Ԫ���غ��֪n��Al��=n[Al��OH��3]=0.4mol��n��Mg��=n[Mg��OH��2]=0.3mol����Mg��Al��������Ϊ0.4mol��27g/mol+0.3mol��24g/mol=18g��
���������ʱΪMg��OH��2��Al��OH��3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn��NaOH��=2n��Na2SO4��=0.4L��5mol/L=2mol������n��Na2SO4��=1mol�����������Ũ��Ϊ
| 1mol |
| 0.4L |
�ɣ�1���п�֪n��Al��=0.4mol��n��Mg��=0.3mol�����ݵ���ת���غ��֪2n��H2��=3n��Al��+2n��Mg��=3��0.4mol+2��0.3mol=1.8mol������n��H2��=0.9mol��
�ʴ�Ϊ��18g��2.5mol/L��0.9mol��
���������⿼�黯ѧ����ʽ�йؼ��㡢�������㣬����ƴ������Ŀ�����ؿ���ѧ���������������������Ŀ�������ϴ�ѧ�������������нϸߵ�Ҫ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
������ˮ�⣬��ˮ�ⲻ��ȫ�������ǣ�������
| A�����ܷ���������Ӧ������ʹ����Һ����ɫ |
| B�����ܷ���������Ӧ�����ܺ�����Cu��OH��2��Ӧ���ɺ�ɫ���� |
| C��������ζ���ֲ����� |
| D������ʹ����Һ������ȴ����������Ӧ |
2005����ף�һ�������������Դ��п-��ȼ�ϵ�ء�Ϊ����װ���ġ����۸ɾ����������糵���������Ϻ��ֶ���ͷ����Ͷ�������нΣ���п-��ȼ�ϵ�ء��ǽ���-������ص�һ�֣����ڰ�ȼ�ϵ�أ����������ߡ�ԭ���Ϸḻ���۸�͵��ŵ㣮�����йء�п-��ȼ�ϵ�ء���˵��������ǣ�������
| A���õ�ؿ��ܱ��㷺ʹ�� |
| B���õ�ز�����ʱ����Ӧ�����ܷ�� |
| C���õ��ʹ�ú�����Ի��������Ⱦ |
| D���õ�صĸ�����ӦΪ��2Zn+4OH-��2ZnO+2H2O+4e- |
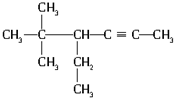
�����л���������ͬ����������ǣ�������
| A������Ķ��ȴ��� |
| B�����ȱ� |
| C���춡���һ�ȴ��� |
| D���������һ�ȴ��� |
��������ˮ��Һ���ܴ���������ǣ�������
| A��Fe3+��H+��I-��Na+ |
| B��Al3+��Na+��AlO2-��SO42- |
| C��K+��NH4+��H+��CO32- |
| D��Cu2+��SO42-��Al3+��Cl- |
 ��ϵͳ����Ϊ
��ϵͳ����Ϊ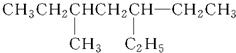 ��
�� ��
��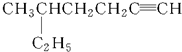
 ��
��