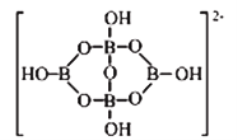
��Ŀ����
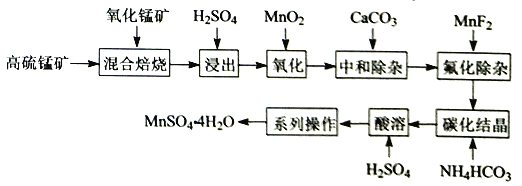
����Ŀ������һ�ְ뵼����ϣ�����ϡɢ����������Ϊ���ִ���ҵ���������˼�����ά���أ������˼��漣�����������ǵ��������²��ϵ�֧�Ų��ϡ����ڻ���ͭ������Ҫ����ΪCu2Te��Cu��CuSO45H2O��Au��Ag�ȣ�Ϊԭ����ȡ���Ʊ�TeO2�͵���Te�Ĺ���������ͼ��ʾ��
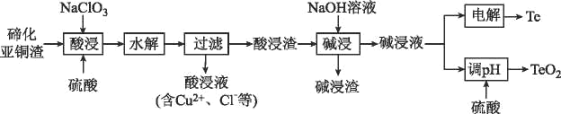
��֪����ˮ������ӦΪH2TeO3(������)=TeO2��+H2O��
�ش��������⣺
��1��Cu2Te��Te�Ļ��ϼ�Ϊ___��
��2���������ʱ��Ҫʹ6molCu�ܽ⣬��Cu��Ӧ��NaC1O3�����ʵ���Ϊ___��
��3��д���������ʱCu2Te����ת�������ӷ���ʽ��___��
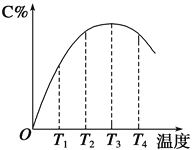
��4��ȡ�ڻ���ͭ��100g����������������������Ũ�ȶ��ڻ���ͭ������Ч����Ӱ����ͼ��ʾ��
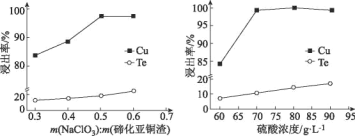
ѡ����ѵ���������������Ϊ___g��ѡ�������Ũ��ԼΪ___mol/L(����С�����һλ����
��5������������к��еĽ���������Ҫ��___(�ѧʽ�������кܸߵľ������ü�ֵ��
��6�������Һ���������������ҺpH��5.5��������TeO2���ù��̵����ӷ���ʽΪ____��
��7����������ǹ�ҵ���Ʊ���Te�ij��÷������Բ���ְ����ͨ������������������һ���ĵ����ܶȡ��¶��µ����Һ����Ԫ���Խ���Te��ʽ�������������������ĵ缫��ӦʽΪ___��
���𰸡�-2 2mol Cu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2O 50 0.7 Au��Ag TeO32-+2H+= TeO2��+ H2O TeO32-+ 3H2O+ 4e-=Te+6OH-
��������
�ڻ���ͭ������Ҫ����ΪCu2Te��Cu��CuSO45H2O��Au��Ag�ȣ��������������ܽ⣬����Cu��Au��Ag�������ᷴӦ���γɺ���Cu+��Cu2+��Te2+��SO42-����Һ���ټ��������ƣ���������������������Ӿ���ǿ�����ԣ�����Һ��Cu��Cu+����ΪCu2+��Te2+������ת��ΪH2TeO3������ˮ��H2TeO3ת��ΪTeO2�����˺������Һ�к���Cu2+��Cl-���������ijɷ�ΪAu��Ag��TeO2�����������м����������ƣ���TeO2�ܽ�ת��Na2TeO3���ڹ��ˣ��õ�������ͼ��Һ�����������Ҫ����Au��Ag�����Һ��ҪΪNa2TeO3���Լ��Һ���ɵõ�Te������Һ�м����������pHֵ����TeO2���ݴ˷������
(1)�ڻ���ͭCu2Te��Cu Ϊ+1�ۣ�Te���ϼ�Ϊ-2�ۣ�
(2) 6molCuת��ΪCu2+ʧȥ12mole-��1mol NaC1O3ת��ΪCl-�õ�6mol e-����Ҫʹ6molCu�ܽ⣬��Cu��Ӧ��NaC1O3�����ʵ���Ϊ2mol��
(3)д���������ʱ����������������������Ӿ���ǿ�����ԣ� Te2+������ת��ΪH2TeO3��Cu2Te����ת�������ӷ���ʽ��Cu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2O��
(4)ͭ���ڵĽ��������������������������Ӷ�����m(NaC1O3): m(�ڻ���ͭ��)��0.5ʱ��ͭ�����ʱ仯�����ڵĽ����ʴ�������ӣ���ˣ�Ϊ�����ڵ���ʧ��ͭ����Ч���룬ѡ��m(NaC1O3): m(�ڻ���ͭ��)=0.5���ڻ���ͭ��Ϊ100g����NaC1O3��������Ϊ50g��ͭ���ڵĽ�������������Ũ�ȵ���������ߣ�������Ũ������70g/Lʱ��������������Ũ�ȶ�ͭ�Ľ�����Ӱ�첻���������ٽ��ڵ��ܽ⣬��ˣ�ѡ����������Ũ��Ϊ70g/L�����ʵ���Ũ��ԼΪ![]() ��0.7mol/L��
��0.7mol/L��
(5)���ݷ�����Au��Ag�����ȶ����������ᡢ����������Һ��Ӧ���� ����������к��еĽ���������Ҫ��Au��Ag��
(6)���ݷ��������Һ��ҪΪNa2TeO3ͨ�����������ҺpH�����е����ӷ�Ӧ����ʽΪTeO32-+2H+= TeO2��+ H2O��
(7)�����Һ����Ԫ���Խ���Te��ʽ�������������������ĵ缫��ӦʽΪTeO32-+3H2O+ 4e-=Te+6OH-��

����Ŀ����������ʵ�����������ó��Ľ�����ȷ����( )
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ������ | ������ʹʪ�����ɫʯ����ֽ��� | �ȴ�����ˮ�е���� |
B | ���������ᴦ���� | �����ɳ�ɫ��Ϊ��ɫ | �Ҵ����������� |
C | �� | ������ɫ���� | ������������� |
D | ����������ȼ�ճ��е�ȼ��Ѹ�����뼯�� | �����������̣�ƿ���к�ɫ�������� |
|
A.AB.BC.CD.D