��Ŀ����
��Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
(1)д����Ӧ�����ӷ���ʽ�� ��
(2)������������£����ӷ���ʽ�� (1)��ͬ���� (�����)��
A����NaHSO4��Һ����μ���Ba(OH)2��Һ����Һ������
B����NaHSO4��Һ����μ���Ba(OH)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(OH)2��Һ������
(3)����������ϡ����ֱ�����������������л����Һ�ĵ�������(�õ���ǿ��I��ʾ)�ɽ��Ƶ�����ͼ�е� (�����)���߱�ʾ��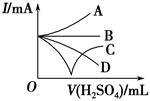
(4)����һ����⻬������С��������Ba(OH)2��Һ���룬��ͼ��ʾ������ձ��л���ע����Ba(OH)2��Һ���ܶȵ�ϡ������ǡ����ȫ��Ӧ���ڴ�ʵ������У�С�� ��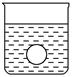
(1)Ba2����2OH����2H����SO42��=BaSO4����2H2O��(2)A��(3)C��(4)�³�
����

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д� �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�������ط�Ӧ�����ӷ���ʽ��д��ȷ����
| A������������������Fe(OH)3+3H+ = Fe3++3H2O |
| B������ͭ��Һ�����ԣ�Cu2+ + 2H2O = Cu(OH)2��+ 2H+ |
C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ�NH4++OH-  NH3��+H2O NH3��+H2O |
| D�����ữ�ĸ��������Һ����˫��ˮ��2MnO4-+6H++5H2O2 = 2Mn2++5O2��+8H2O |
����ѧ����ѡ��2����ѧ�뼼������15�֣�
��Ƴ���ͭ��ˮ�к���CN-��Cr2O72-���ӣ���Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ�������ش��������⣺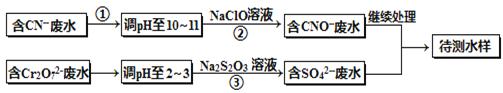
��1������������ˮ��������Ҫʹ�õķ�����_________________��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ______________��
��3��������У�ÿ����0.4mol Cr2O72-ʱת�Ƶ���2.4mol���÷�Ӧ�����ӷ���ʽΪ ��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ����ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ�� ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4 ��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�ڣ� ����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����1mol Cr2O72-�������a mol FeSO4 ? 7H2O�����н�����ȷ����_______��
| A��x ="0.5" ,a ="8" | B��x ="0.5" ,a =" 10" | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
��������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
(1)��ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������________��
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� |
| C��ֻ�������� | D�������Ժ����� |
(2)Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
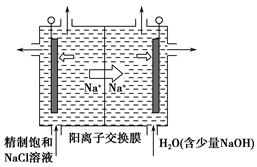
����ͼ����ʯī���缫��һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��ʾ��ͼ������������ClO2�ĵ缫��ӦʽΪ_____________________________________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ________mol����ƽ���ƶ�ԭ������������pH�����ԭ��______________________________________��
(3)ClO2����ˮ��Fe2����Mn2����S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�CN��a mg/L������ClO2��CN���������������������������ɣ������ӷ�Ӧ����ʽΪ________________������100 m3������ˮ��������ҪClO2________mol��
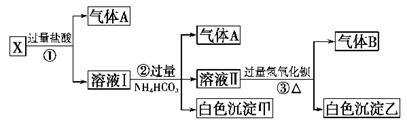
 (NH4)2Fe(SO4)2 ��Һ��ַ�Ӧ������Fe2+����0��0500 mol��
(NH4)2Fe(SO4)2 ��Һ��ַ�Ӧ������Fe2+����0��0500 mol��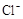 +5Fe3+ +2H2O 14H+ +
+5Fe3+ +2H2O 14H+ +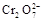 +6 Fe2+ =2Cr3+ + 6 Fe3+ +7H2O
+6 Fe2+ =2Cr3+ + 6 Fe3+ +7H2O