��Ŀ����
����Ŀ���������а����л��
�������а����л����CH4����CH3CH2OH����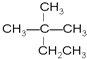 ���ܹ��飬��CH3COOH����
���ܹ��飬��CH3COOH����![]() ����
����![]() �������
�������
�����������ʣ��ش��������⣺
(1)��Է�������Ϊ44�������Ľṹ��ʽΪ___________________��
(2)������Ϊͬ���칹�����__________![]() �����
�����![]() ��
��
(3)����������ζ��������ȡ����ij�л�����FeBr3�������������¿���Һ�巢��ȡ����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ________________��
(4)�л������ڼ��������º�CuO��Ӧ�Ļ�ѧ����ʽΪ______________________��
(5)��120����1.01��105Pa�����£�ij����̬����������O2��ȫ��Ӧ��÷�Ӧǰ����������û�з����ı䣬�������__________![]() �����
�����![]() ��
��
���𰸡�![]()
![]()
![]() +Br2
+Br2![]()
![]() +HBr
+HBr ![]()
![]()
��������
(1)����������ͨʽCnH2n+2�����㣻
(2)����ʽ��ͬ���ṹ��ͬ���л��ﻥΪͬ���칹�壻
(3)����������ζ��������ȡ�������л�����Һ�巢��ȡ����Ӧ����Ϊ���������嵥�ʷ�Ӧ�����屽��
(4)�Ҵ���CuO��Ӧ������ȩ��Cu��ˮ��
(5)��������ˮΪ���壬��Ӧǰ����������û�з����ı䣬��ǰ������Ļ�ѧ��������ȣ������������������г��ȼ�յ�ͨʽ���㣻
(1) ������ͨʽΪ��CnH2n+2����Է�������Ϊ44����������12n+2n+2=44������n=3���������ķ���ʽΪC3H8���ṹ��ʽΪCH3CH2CH3��
�ʴ�Ϊ��CH3CH2CH3��
(2)��ۻ�Ϊͬ���칹����Ǣߣ����߷���ʽ��ͬ���ṹ��ͬ��
�ʴ�Ϊ���ߣ�
(3)����������ζ��������ȡ�����л���Ϊ������FeBr3����������������Һ�巢��ȡ����Ӧ�Ļ�ѧ����ʽΪ![]() +Br2
+Br2![]()
![]() +HBr��
+HBr��
�ʴ�Ϊ��![]() +Br2
+Br2![]()
![]() +HBr��
+HBr��
(4)�Ҵ��ڼ��������º�CuO��Ӧ������ȩ��Cu��ˮ����ѧ����ʽΪCH3CH2OH+CuO��CH3CHO+Cu+H2O���ʴ�Ϊ��CH3CH2OH+CuO��CH3CHO+Cu+H2O��
(5)��120����1.01��105Pa�������£����ɵ�ˮΪ��̬����CxHy+(x+![]() )O2��xCO2+
)O2��xCO2+![]() H2O(g)����1+(x+
H2O(g)����1+(x+![]() )=x+
)=x+![]() �����
�����![]() ��������ʽ����ԭ����ĿΪ4��Ϊ���飬
��������ʽ����ԭ����ĿΪ4��Ϊ���飬
�ʴ�Ϊ���١�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�