��Ŀ����
��֪Cl2�ͼ���Һ�ڲ�ͬ�����£��õ��IJ��ﲻͬ��ij��ȤС������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ���3Cl2+6KOH KClO3+5KCl+3H2O ��
KClO3+5KCl+3H2O ��
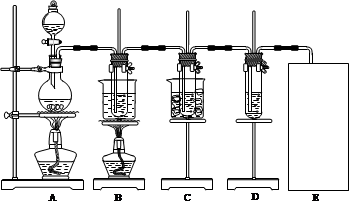
ͼ�У�AΪ��������װ�ã�B���Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У�C���Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У�D���Թ��������ɫʯ����Һ������д���пհף�
��1����ȡ����ʱ����Բ����ƿ�����һ�������Ķ������̣�ͨ�� (����������)��Բ����ƿ�м���������Ũ���ᡣװ��A�з�Ӧ�����ӷ���ʽΪ ����Ҫ����ƿ�м����Ƭ�� ��ѡ�����Ҫ����������Ҫ������
��2����Ӧ��������ƿ�����Һ ��
Aһ�������ԣ�B���������ԣ�Ҳ����Ϊ���ԣ�Cֻ�������ԣ�Dֻ�л�ԭ�ԣ�E�������������л�ԭ��
��3����Ӧ��ϣ�B�Թ�����������������������ȴ���д�������������ͼ�з��ϸþ����ܽ�����ߵ��� (������ĸ)����B���Թ��з�����þ�������õ��IJ��������� ��
��4����С��ͬѧ�����Ƶõ�����ز���ƫ�ͣ����ܵ�һ��ԭ����Cl2�к���HCl���塣�Դ��������ͨ���Ľ�ʵ��װ�õķ������б��⡣������ ��
��5��ʵ���пɹ۲쵽D���Թ�����Һ����ɫ����ɫ�ȱ�Ϊ_________ɫ�����ձ�Ϊ________ɫ��
��6��Cװ���з�Ӧ��ϵ�������______________________________________________��
��7������װ��ͼ�����л���ȱ�ٵ�ʵ��װ�ã���ע���Լ���
 KClO3+5KCl+3H2O ��
KClO3+5KCl+3H2O ��
ͼ�У�AΪ��������װ�ã�B���Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У�C���Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У�D���Թ��������ɫʯ����Һ������д���пհף�
��1����ȡ����ʱ����Բ����ƿ�����һ�������Ķ������̣�ͨ�� (����������)��Բ����ƿ�м���������Ũ���ᡣװ��A�з�Ӧ�����ӷ���ʽΪ ����Ҫ����ƿ�м����Ƭ�� ��ѡ�����Ҫ����������Ҫ������
��2����Ӧ��������ƿ�����Һ ��
Aһ�������ԣ�B���������ԣ�Ҳ����Ϊ���ԣ�Cֻ�������ԣ�Dֻ�л�ԭ�ԣ�E�������������л�ԭ��
��3����Ӧ��ϣ�B�Թ�����������������������ȴ���д�������������ͼ�з��ϸþ����ܽ�����ߵ��� (������ĸ)����B���Թ��з�����þ�������õ��IJ��������� ��
��4����С��ͬѧ�����Ƶõ�����ز���ƫ�ͣ����ܵ�һ��ԭ����Cl2�к���HCl���塣�Դ��������ͨ���Ľ�ʵ��װ�õķ������б��⡣������ ��
��5��ʵ���пɹ۲쵽D���Թ�����Һ����ɫ����ɫ�ȱ�Ϊ_________ɫ�����ձ�Ϊ________ɫ��
��6��Cװ���з�Ӧ��ϵ�������______________________________________________��
��7������װ��ͼ�����л���ȱ�ٵ�ʵ��װ�ã���ע���Լ���
��Һ©����1�֣���MnO2+4H++2Cl- Mn2++Cl2��+2H2O��2�֣�����ѧʽ����δ��ƽ�֡���������Ӧ�������������ؼ���ͷ���Ⱥ��óɼ�ͷ�������ţ��ϼƿ�1�֡��˱���ͬ��������Ҫ��1�֣�������ѡ�������������˵���������֣����籾������������֡��˱���ͬ��
Mn2++Cl2��+2H2O��2�֣�����ѧʽ����δ��ƽ�֡���������Ӧ�������������ؼ���ͷ���Ⱥ��óɼ�ͷ�������ţ��ϼƿ�1�֡��˱���ͬ��������Ҫ��1�֣�������ѡ�������������˵���������֣����籾������������֡��˱���ͬ��
��2��AE��2�֣���ȫ�Ե�2�֣��Զ���ȫ��1�֣�����÷֣�
��3��M��1�֣���©��������ͨ©��������©������������������дΪ���������ձ�����С�ձ�����1�֣� ��ȫ�Բ��ܵ�1�֣�
��4����A��Bװ��֮���һ��ʢ����ʳ��ˮ���Թܣ���ϴ��ƿ���Լ�ƿ����2�֣���λ��1�֡��Լ�1�֡������ɷ֡�ij�������Ӱ����һ��÷֡�λ�ã�A���Bǰ���÷֡��Լ������ͱ����У�NaCl�����������ܽ�Ƚϴ�������Ρ���
��5���죨1�֣����ޣ�1�֣� ��6��C���Թ��ϲ��ռ��������ɫ���壨1�֣����������𰸸��֣�
��7�� ���Լ�Ϊǿ����Һ��Ũ�Ȳ���Ҫ��1�֣����ڵ���Ҫͨ��Һ������1/3��̫dz���� �� ��һ��������ϵ����1�֣����ٺ͢ڢ۷ֿ��ɷ֣��˴˲�ǣ�����ڢ۴�һ�������2�ֲ�������
���Լ�Ϊǿ����Һ��Ũ�Ȳ���Ҫ��1�֣����ڵ���Ҫͨ��Һ������1/3��̫dz���� �� ��һ��������ϵ����1�֣����ٺ͢ڢ۷ֿ��ɷ֣��˴˲�ǣ�����ڢ۴�һ�������2�ֲ�������
 Mn2++Cl2��+2H2O��2�֣�����ѧʽ����δ��ƽ�֡���������Ӧ�������������ؼ���ͷ���Ⱥ��óɼ�ͷ�������ţ��ϼƿ�1�֡��˱���ͬ��������Ҫ��1�֣�������ѡ�������������˵���������֣����籾������������֡��˱���ͬ��
Mn2++Cl2��+2H2O��2�֣�����ѧʽ����δ��ƽ�֡���������Ӧ�������������ؼ���ͷ���Ⱥ��óɼ�ͷ�������ţ��ϼƿ�1�֡��˱���ͬ��������Ҫ��1�֣�������ѡ�������������˵���������֣����籾������������֡��˱���ͬ����2��AE��2�֣���ȫ�Ե�2�֣��Զ���ȫ��1�֣�����÷֣�
��3��M��1�֣���©��������ͨ©��������©������������������дΪ���������ձ�����С�ձ�����1�֣� ��ȫ�Բ��ܵ�1�֣�
��4����A��Bװ��֮���һ��ʢ����ʳ��ˮ���Թܣ���ϴ��ƿ���Լ�ƿ����2�֣���λ��1�֡��Լ�1�֡������ɷ֡�ij�������Ӱ����һ��÷֡�λ�ã�A���Bǰ���÷֡��Լ������ͱ����У�NaCl�����������ܽ�Ƚϴ�������Ρ���
��5���죨1�֣����ޣ�1�֣� ��6��C���Թ��ϲ��ռ��������ɫ���壨1�֣����������𰸸��֣�
��7��
 ���Լ�Ϊǿ����Һ��Ũ�Ȳ���Ҫ��1�֣����ڵ���Ҫͨ��Һ������1/3��̫dz���� �� ��һ��������ϵ����1�֣����ٺ͢ڢ۷ֿ��ɷ֣��˴˲�ǣ�����ڢ۴�һ�������2�ֲ�������
���Լ�Ϊǿ����Һ��Ũ�Ȳ���Ҫ��1�֣����ڵ���Ҫͨ��Һ������1/3��̫dz���� �� ��һ��������ϵ����1�֣����ٺ͢ڢ۷ֿ��ɷ֣��˴˲�ǣ�����ڢ۴�һ�������2�ֲ������������������1������ƿ�м���Ũ���ᣬ��Ҫ�����ڷ�Һ©�����ڼ��ȵ������¶�����������Ũ����������������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl-
 Mn2++Cl2��+2H2O�������ڷ�Ӧ�ж������̲�����ˮ���Թ������ʽ���ڣ���˷�Ӧ�в���Ҫ�ټ������Ƭ��
Mn2++Cl2��+2H2O�������ڷ�Ӧ�ж������̲�����ˮ���Թ������ʽ���ڣ���˷�Ӧ�в���Ҫ�ټ������Ƭ����2�������ڷ�Ӧ������Ũ�����Ũ�����ͣ����������̲�������ϡ���ᣬ���Է�Ӧ����������һ��ʣ�࣬��Һ�����ԡ�ͬʱ��Ӧ�л������Ȼ��̾��л�ԭ�ԡ�����Һ�е������ӻ����������ԣ����Դ�ѡAE��
��3����ȴ���д���������������˵�������ʵ��ܽ�����¶ȵ�Ӱ��ϴ����ܽ�����¶ȵ����߶��������Է���������������M������Һ�з��������IJ����ǹ��ˣ���Ҫ�IJ���������©�������������ձ���
��4�������Ȼ��⼫������ˮ��������ˮ�е��ܽ�Ƚ�С������Ҫ��ȥ�����е��Ȼ������壬����ͨ�뵽ʢ�б���ʳ��ˮ��ϴ��ƿ�У�����ȷ��������A��Bװ��֮���һ��ʢ����ʳ��ˮ��ϴ��ƿ��
��5����������ˮ��������������ᡣ��Һ�����ԣ���ʯ����Һ��Ϊ��ɫ������Ϊ�����ỹ����ǿ�����ԣ���Ư�����ָʾ���������Һ���ձ�Ϊ��ɫ��
��6���ڱ�ˮԡ������������������Һ�ķ�Ӧ��������������ˮ�е��ܽ�Ⱥ�С������Cװ���з�Ӧ��ϵ�������C���Թ��ϲ��ռ��������ɫ���塣
��7�������ж�����Ҫβ��������һ��������������Һ���գ���ʵ��װ��ͼΪ
 ��
��
��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ
 MnCl2+2H2O+Cl2��
MnCl2+2H2O+Cl2�� CaCl2+2H2O
CaCl2+2H2O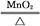 2KCl+3O2��
2KCl+3O2��
