��Ŀ����
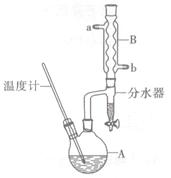
��������һ����Ҫ�Ļ����ϳ�ԭ�ϡ�ʵ���Һϳ�������ķ�Ӧ��ʵ��װ�����£�
��Ӧ��CH3CH2OH+HBr CH3CH2Br+H2O
CH3CH2Br+H2O
װ�ã�
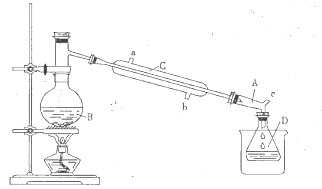
ʵ���п����õ����������±���
�ٺϳɷ�Ӧ��������B�м�������NaBr��Ũ�����50mL�Ҵ�����װ���������������ȣ��ռ�����
�ڷ����ᴿ����������ˮ�����ټ����������ȥ���ѣ���Һ�����õ�52mL�����顣
�ش��������⣺
��1������B��������___________��
��2������CΪֱ�������ܣ���ˮ������___________���a����b������
��3������D����ʢ��_________���ձ��У�Ŀ����__________________________________��
��4��װ��A������Ϊţ�ǹܣ���ṹ�е�c֧�ܵ�������___________________________��
��5��������ֲ�Ʒ�����ᴿ�Σ���ˮ��Ŀ����___________________________________��
��6����ʵ������������IJ���Ϊ ________________________��
��Ӧ��CH3CH2OH+HBr
 CH3CH2Br+H2O
CH3CH2Br+H2Oװ�ã�

ʵ���п����õ����������±���
| ���� | ��Է������� | �е�/�� | �ܶ�/g/cm3 | ˮ���� |
| CH3CH2OH | 46 | 78.4 | 0.79 | ���� |
| CH3CH2Br | 109 | 38.4 | 1.42 | ���� |
| CH3CH2OCH2CH3 | 74 | 34.5 | 0.71 | �� |
| CH2=CH2 | 28 | -103.7 | 0.38 | ���� |
| Ũ���ᣨH2SO4�� | 98 | 338.0 | 1.38 | ���� |
�ٺϳɷ�Ӧ��������B�м�������NaBr��Ũ�����50mL�Ҵ�����װ���������������ȣ��ռ�����
�ڷ����ᴿ����������ˮ�����ټ����������ȥ���ѣ���Һ�����õ�52mL�����顣
�ش��������⣺
��1������B��������___________��
��2������CΪֱ�������ܣ���ˮ������___________���a����b������
��3������D����ʢ��_________���ձ��У�Ŀ����__________________________________��
��4��װ��A������Ϊţ�ǹܣ���ṹ�е�c֧�ܵ�������___________________________��
��5��������ֲ�Ʒ�����ᴿ�Σ���ˮ��Ŀ����___________________________________��
��6����ʵ������������IJ���Ϊ ________________________��
��1��������ƿ
��2��b
��3����ˮ����� ��ȴ������
��4��ƽ��װ��D װ��B�е���ѹ������װ��D�������Һ
��5����ȥ�ܽ����������е��Ҵ�
��6��78.9%��79%��78.89%
��2��b
��3����ˮ����� ��ȴ������
��4��ƽ��װ��D װ��B�е���ѹ������װ��D�������Һ
��5����ȥ�ܽ����������е��Ҵ�
��6��78.9%��79%��78.89%
�����������1������B��������������ƿ����2��Ϊ��ʹ����Ч�����ã�����Cֱ�������ܣ���ˮ������b.��ˮ����Ϊa����3��������ķе�Ϊ38.4�㣬Ϊ���ռ���Ʒ��Ҫ������D����ʢ�б�ˮ�������ձ��У�����ȴ�����顣��4��װ��A������Ϊţ�ǹܣ���ṹ�е�c֧�ܵ�������ƽ��װ��D��װ��B�е���ѹ������װ��D�������Һ����5�������Ҵ��ķе�ֻ��78.4�㣬�ڼ���ʱ��������Ӧ�������������������Ҵ���ˮ���ܣ��������鲻���ܽ���ˮ��������������ֲ�Ʒ�����ᴿ�Σ���ˮ�Ϳ��Գ�ȥ�ܽ����������е��Ҵ������ڻ����ķ��롣��6��n(�Ҵ�)="(" 50mL��0.79g/ml)��46g/mol=0.8587mol;n(������)= (52mL��1.42g/ml)��109g/mol=0.6774mol.���Ը�ʵ������������IJ���Ϊ(0.6774��0.8587)��100%=78.9%.

��ϰ��ϵ�д�
�����Ŀ
 (CH3CH2CH2CH2)2O+H2O
(CH3CH2CH2CH2)2O+H2O