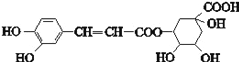��Ŀ����
����Ŀ����A��B��C��D��E�������������������ʣ�
�ٸ�ȡ0.1mol�ֱ���ȼ�գ�����B��C��Eȼ�����õ�CO2��Ϊ4.48L(��״��)��A��Dȼ�����õ�CO2����ǰ���ߵ�3����
�������������£�A��B��C���ܸ����������ӳɷ�Ӧ������A����ת��ΪD��B����ת��ΪC��C����ת��ΪE��
��B��C����ʹ��ˮ������KMnO4��Һ��ɫ����A��D��E�����ʣ�
������м������ʱ��A�����巢��ȡ����Ӧ��
(1)�ж�A��B��D��E����ʲô���ʣ�д���������ʵĽṹ��ʽ��A_______B_______C_______D________E________��
(2)����E�����ȴ�����______�֡�
(3)д��C��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��_______
(4)д�������漰�Ļ�ѧ����ʽ��__________
���𰸡�![]() CH��CH CH2=CH2
CH��CH CH2=CH2 ![]() CH3CH3 2 CH2=CH2+Br2��CH2BrCH2Br
CH3CH3 2 CH2=CH2+Br2��CH2BrCH2Br ![]() +Br2
+Br2![]()
![]() +HBr
+HBr
��������
ȡ0.1mol�������ֱ�ȼ�գ�����CO2�������ж�B��C��E��Cԭ�Ӹ���Ϊ2��A��D��Cԭ�Ӹ���Ϊ6���ٸ���A��B��C���ܸ����������ӳɷ�Ӧ������A����ת��ΪD��B����ת��ΪC��C����ת��ΪE��B��C����ʹ��ˮ������KMnO4��Һ��ɫ����A��D��E�����ʣ��Ϳ�һһ���
��ȡ0.1mol���������ֱ�ʹ֮���ȼ�գ�����B��C��Eȼ�����õ�CO2��Ϊ4.48L���������������̼�����ʵ���= ![]()
![]() =0.2mol������B��C��E�����к���2��̼ԭ�ӣ�A��Dȼ�����õ�CO2����B��C��E����������A��D�����к���6��̼ԭ�ӣ����ʵ��������£�A��B��C���ܸ����������ӳɷ�Ӧ��˵�����в����ͼ���������A��ֱ��ת��ΪD��B��ת��ΪC��C��ת��ΪE��B��C����ʹ��ˮ�����Ը��������Һ��ɫ����A��D��E�������ʣ�����֪B����̼̼������C�к���̼̼˫��������B����Ȳ��C����ϩ��E�����飻����м������ʱ��A����Һ̬�巢��ȡ����Ӧ��A����6��̼ԭ�ӣ���Ϊ������A�DZ���A��ת��ΪD����D�ǻ����飬
=0.2mol������B��C��E�����к���2��̼ԭ�ӣ�A��Dȼ�����õ�CO2����B��C��E����������A��D�����к���6��̼ԭ�ӣ����ʵ��������£�A��B��C���ܸ����������ӳɷ�Ӧ��˵�����в����ͼ���������A��ֱ��ת��ΪD��B��ת��ΪC��C��ת��ΪE��B��C����ʹ��ˮ�����Ը��������Һ��ɫ����A��D��E�������ʣ�����֪B����̼̼������C�к���̼̼˫��������B����Ȳ��C����ϩ��E�����飻����м������ʱ��A����Һ̬�巢��ȡ����Ӧ��A����6��̼ԭ�ӣ���Ϊ������A�DZ���A��ת��ΪD����D�ǻ����飬
(1)������������֪��AΪ![]() ��BΪCH��CH��CΪCH2=CH2��DΪ
��BΪCH��CH��CΪCH2=CH2��DΪ ![]() ��EΪCH3CH3 ��
��EΪCH3CH3 ��
�ʴ�Ϊ��AΪ![]() ��BΪCH��CH��CΪCH2=CH2��DΪ
��BΪCH��CH��CΪCH2=CH2��DΪ ![]() ��EΪCH3CH3 ��
��EΪCH3CH3 ��
(2) EΪCH3CH3�������ȴ���ΪCCl3CH2Cl��CHCl2CHCl2����2�ֽṹ��
�ʴ�Ϊ��2��
(3) C��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br��
(4) �����漰�Ļ�ѧ����ʽΪ��![]() +Br2
+Br2![]()
![]() +HBr��
+HBr��
�ʴ�Ϊ��![]() +Br2
+Br2![]()
![]() +HBr��
+HBr��