题目内容
(14分)化学兴趣小组对某品牌牙膏中摩擦剂成分及其含量进行以下探究:
查得资料:该牙膏摩擦剂由碳酸钙、氢氧化铝组成;牙膏中其他成分遇到盐酸时无气体产生。
Ⅰ.摩擦剂中氢氧化铝的定性检验。
取适量牙膏样品,加水充分搅拌、过滤。
(1)往滤渣中加入过量NaOH溶液,过滤。氢氧化铝与NaOH溶液反应的离子方程式是______________________________。
(2)往(1)所得滤液中先通入过量二氧化碳,再加入过量稀盐酸。观察到的现象是__________________________________________________________。
Ⅱ.牙膏样品中碳酸钙的定量测定。
利用如图所示装置(图中夹持仪器略去)进行实验,充分反应后,测定C中生成的BaCO3沉淀质量,以确定碳酸钙的质量分数。
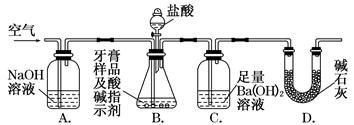
依据实验过程回答下列问题:
(3)实验过程中需持续缓缓通入空气。其作用除了可搅拌B、C中的反应物外,还有__________________________________________
(4)C中反应生成BaCO3的化学方程式是_____________________________________
(5)下列各项措施中,不能提高测定准确度的是________(填标号)。
a.在加入盐酸之前,应排净装置内的CO2气体
b.滴加盐酸不宜过快
c.在A~B之间增添盛有浓硫酸的洗气装置
d.在B~C之间增添盛有饱和碳酸氢钠溶液的洗气装置
(6)实验中准确称取8.00 g样品三份,进行三次测定,测得BaCO3平均质量为3.94 g。则样品中碳酸钙的质量分数为________。
(7)有人认为不必测定C中生成的BaCO3质量,只要测定装置C在吸收CO2前后的质量差,就可以确定碳酸钙的质量分数。实验证明按此方法测定的结果明显偏高,原因是______________________________。
查得资料:该牙膏摩擦剂由碳酸钙、氢氧化铝组成;牙膏中其他成分遇到盐酸时无气体产生。
Ⅰ.摩擦剂中氢氧化铝的定性检验。
取适量牙膏样品,加水充分搅拌、过滤。
(1)往滤渣中加入过量NaOH溶液,过滤。氢氧化铝与NaOH溶液反应的离子方程式是______________________________。
(2)往(1)所得滤液中先通入过量二氧化碳,再加入过量稀盐酸。观察到的现象是__________________________________________________________。
Ⅱ.牙膏样品中碳酸钙的定量测定。
利用如图所示装置(图中夹持仪器略去)进行实验,充分反应后,测定C中生成的BaCO3沉淀质量,以确定碳酸钙的质量分数。

依据实验过程回答下列问题:
(3)实验过程中需持续缓缓通入空气。其作用除了可搅拌B、C中的反应物外,还有__________________________________________
(4)C中反应生成BaCO3的化学方程式是_____________________________________
(5)下列各项措施中,不能提高测定准确度的是________(填标号)。
a.在加入盐酸之前,应排净装置内的CO2气体
b.滴加盐酸不宜过快
c.在A~B之间增添盛有浓硫酸的洗气装置
d.在B~C之间增添盛有饱和碳酸氢钠溶液的洗气装置
(6)实验中准确称取8.00 g样品三份,进行三次测定,测得BaCO3平均质量为3.94 g。则样品中碳酸钙的质量分数为________。
(7)有人认为不必测定C中生成的BaCO3质量,只要测定装置C在吸收CO2前后的质量差,就可以确定碳酸钙的质量分数。实验证明按此方法测定的结果明显偏高,原因是______________________________。
(1)Al(OH)3+OH-===AlO2-+2H2O或Al(OH)3+OH-===[Al(OH)4]-
(2)通入CO2气体有白色沉淀生成;加入盐酸有气体产生、沉淀溶解
(3)把生成的CO2全部排入C中,使之完全被Ba(OH)2溶液吸收
(4)Ba(OH)2+CO2===BaCO3↓+H2O (5)c、d (6)25%
(7)B中的水蒸气、HCl气体等进入装置C中(或其他合理答案)
(2)通入CO2气体有白色沉淀生成;加入盐酸有气体产生、沉淀溶解
(3)把生成的CO2全部排入C中,使之完全被Ba(OH)2溶液吸收
(4)Ba(OH)2+CO2===BaCO3↓+H2O (5)c、d (6)25%
(7)B中的水蒸气、HCl气体等进入装置C中(或其他合理答案)
试题分析:(1)氢氧化铝与NaOH溶液反应的离子方程式是Al(OH)3+OH-===AlO2-+2H2O
(2)向(1)中滤液中通过量的CO2气体有Al(OH)3白色沉淀生成,加入过量盐酸沉淀又溶解。
(3)通入空气还可以把生成的CO2全部排入C中,使之完全被Ba(OH)2溶液吸收。
(4)C中的反应方程式为Ba(OH)2+CO2===BaCO3↓+H2O
(5)在加入盐酸之前,应排净装置内的CO2气体可以提高测定的准确度,a不选;B装置中有酸碱指示剂,说明反应中盐酸的加入量是有限的,为保证准确,滴加过程要慢,b不选;B中装有溶液,因此气体通入前没有必要干燥,c选;在B—C之间连接盛有饱和NaHCO3溶液的洗气瓶会造成Ba(OH)2溶液还可能吸收由挥发出的氯化氢与NaHCO3反应生成的CO2气体,干扰试验,d选。
(6)根据碳元素守恒得:BaCO3—CO2—CaCO3,m(CaCO3)=
 ,CaCO3的质量分数为
,CaCO3的质量分数为 。
。(7)B中的水蒸气、氯化氢等气体进入装置C中,质量差要大于实际产生CO2气体的质量。
点评:本题综合性强,突出对学生分析实验、理解实验、数据处理等能力的考察。

练习册系列答案
相关题目

 AlN + CO(配平)
AlN + CO(配平)