МвДїДЪИЭ
ДїЗ°КАЅзЙП60%µДГѕКЗґУєЈЛ®ЦРМбИЎµДЎЈТСЦЄєЈЛ®МбИЎГѕµДЦчТЄІЅЦиИзПВЈє

ЈЁ1Ј©№ШУЪјУИлКФјБўЩЧчіБµнјБЈ¬УРТФПВјёЦЦІ»Н¬·Ѕ·ЁЈ¬ЗлНкіЙПВБРОКМвЎЈ
·Ѕ·Ё | КЗ·сХэИ· | јтКцАнУЙ |
·Ѕ·Ё1ЈєЦ±ЅУНщєЈЛ®ЦРјУИліБµнјБ | І»ХэИ· | ЈЁТ»Ј© |
·Ѕ·Ё2ЈєёЯОВјУИИХф·ўєЈЛ®єуЈ¬ФЩјУИліБµнјБ | ЈЁ¶юЈ© | ЈЁИэЈ© |
ДгИПОЄЧоєПАнµДЖдЛы·Ѕ·ЁКЗЈєЎЎЎЎЎЎЈЁЛДЈ© | ||
ЈЁТ»Ј©_______________________________________________Ј»
ЈЁ¶юЈ©_______________________________________________Ј»
ЈЁИэЈ©______________________________________________Ј»
ЈЁЛДЈ©______________________________________________ЎЈ
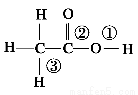
ЈЁ2Ј©їтНјЦРјУИлµДКФјБўЩУ¦ёГКЗ________ЈЁМо»ЇС§КЅЈ©Ј»јУИлµДКФјБўЪКЗ________ЈЁМо»ЇС§КЅЈ©Ј»№¤ТµЙПУЙОЮЛ®MgCl2ЦЖИЎГѕµД»ЇС§·ЅіМКЅОЄ________________________________________ЎЈ
ЈЁ1Ј©ЈЁТ»Ј©єЈЛ®ЦРГѕАлЧУЕЁ¶ИРЎЈ¬іБµнјБУГБїґуЈ¬І»ѕјГ
ЈЁ¶юЈ©І»ХэИ·
ЈЁИэЈ©ДЬФґПыєДґуЈ¬І»ѕјГ
ЈЁЛДЈ©єЈМІЙ№СОєуµГµЅµДїаВ±Л®ЦРЈ¬јУИліБµнјБ
ЈЁ2Ј©CaЈЁOHЈ©2ЎЎHClЎЎMgCl2ЈЁИЫИЪЈ© 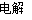 MgЈ«Cl2Ўь
MgЈ«Cl2Ўь
ЎѕЅвОцЎї±ѕМвїјІйµДКЗґУєЈЛ®ЦРМбИЎГѕµДБчіМЎЈКФјБўЩУ¦КЗКЇ»ТИйЈ¬·ўЙъµД·ґУ¦КЗMgCl2Ј«CaЈЁOHЈ©2=MgЈЁOHЈ©2ЎэЈ«CaCl2Ј»КФјБўЪУ¦КЗСОЛбЈ¬·ґУ¦КЗMgЈЁOHЈ©2Ј«2HCl=MgCl2Ј«2H2OЈ¬И»єуЕЁЛхЎўЅбѕ§ЎўНСЛ®µГОЮЛ®MgCl2Ј¬ФЩµзЅвИЫИЪµДMgCl2±гїЙЦЖµГMgЈєMgCl2 ЈЁИЫИЪЈ©  MgЈ«Cl2ЎьЎЈ
MgЈ«Cl2ЎьЎЈ

 У¦УГМвµгІ¦ПµБРґр°ё
У¦УГМвµгІ¦ПµБРґр°ё ЧґФЄј°µЪПµБРґр°ё
ЧґФЄј°µЪПµБРґр°ё Н¬ІЅ°ВКэПµБРґр°ё
Н¬ІЅ°ВКэПµБРґр°ё