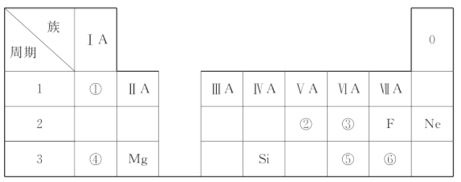
��Ŀ����
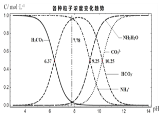
����Ŀ�������£�ijʵ��С��̽��̼�������Һ�и������ʵ���Ũ������ҺpH�ı仯��ͼ��ʾ(������Һ����仯)��������˵��������ǣ� ��
A.��ͼ��֪̼���Ka1������ԼΪ10-7
B.��NH4HCO3��Һ������NH4HCO3�����ʵ���NaOH����Һ��HCO3-������NaOH��Ӧ
C.�����½�NH4HCO3��������ˮ����Һ�Լ���
D.NH4HCO3������ʱ�������ľ�һ���
���𰸡�B
��������
A����ͼ��֪��![]() ʱ��
ʱ��![]() ��
��![]() ��̼���һ������ƽ�ⳣ��
��̼���һ������ƽ�ⳣ��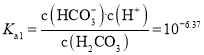 ����̼���
����̼���![]() ������ԼΪ
������ԼΪ![]() ����A��ȷ��
����A��ȷ��
B����ͼ���֪����ͬŨ�ȵ�![]() ��
��![]() ��笠����Ӷ�Ӧ��pHС��˵��
��笠����Ӷ�Ӧ��pHС��˵��![]() ������ʵ���NaOH����Һ���ʱ��
������ʵ���NaOH����Һ���ʱ��![]() ������NaOH��Ӧ����B����
������NaOH��Ӧ����B����
C����ͼ��֪��![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]() �ĵ���ƽ�ⳣ��Ϊ
�ĵ���ƽ�ⳣ��Ϊ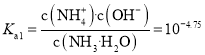 ����̼���
����̼���![]() ����
����![]() �ĵ���������
�ĵ���������![]() ǿ��
ǿ��![]() ��ˮ��̶ȱ�
��ˮ��̶ȱ�![]() ǿ����
ǿ����![]() ��ˮ��Һ�Լ��ԣ���C��ȷ��
��ˮ��Һ�Լ��ԣ���C��ȷ��
D����ľ�ҵ�ˮ��Һ�Լ��ԣ���![]() ���û�����
���û�����![]() ���ͷ�Ч����
���ͷ�Ч����![]() ������ʱ�������ľ�һ��ã���D��ȷ��
������ʱ�������ľ�һ��ã���D��ȷ��
��ѡB��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ