��Ŀ����
��13�֣��±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһԪ�ء�

������ݱ�������Ԫ�أ��ش��������⣺
��1���������δ�ɶԵ���������Ԫ���� ����Ԫ�ط��ţ���1�֣�
��2��f���ʼ�Fe���ʵ��ں��������������������� ����1�֣�
��3��ԭ�Ӻ�p�Dz��������չ����Ԫ��Ϊ ����дԪ�����ƣ�����1�֣���Ԫ����������P����������ˮ����Ļ�ѧ�������ƣ����Դ�������ȥ���գ����� ��д����ҵ�����ø���������ȡ���ʵĻ�ѧ����ʽ ��д������Ԫ���Ȼ����еμ�����Ũ��ˮ�����ӷ���ʽ ��
��4�������ڿ�����ȼ�գ��������ַ�ĩ״���廯�����Ԫ���� ����Ԫ�ط��ţ���
��5����������ᡢ�����������������Ҫ��Ԫ���� ����д������ĸ����

������ݱ�������Ԫ�أ��ش��������⣺
��1���������δ�ɶԵ���������Ԫ���� ����Ԫ�ط��ţ���1�֣�
��2��f���ʼ�Fe���ʵ��ں��������������������� ����1�֣�
��3��ԭ�Ӻ�p�Dz��������չ����Ԫ��Ϊ ����дԪ�����ƣ�����1�֣���Ԫ����������P����������ˮ����Ļ�ѧ�������ƣ����Դ�������ȥ���գ����� ��д����ҵ�����ø���������ȡ���ʵĻ�ѧ����ʽ ��д������Ԫ���Ȼ����еμ�����Ũ��ˮ�����ӷ���ʽ ��
��4�������ڿ�����ȼ�գ��������ַ�ĩ״���廯�����Ԫ���� ����Ԫ�ط��ţ���
��5����������ᡢ�����������������Ҫ��Ԫ���� ����д������ĸ����
��1��N��2���������壨3�� ��
������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ 2Al2O3(����) 4Al+3O2��
4Al+3O2��
Al3++3NH3��H2O=Al(OH)3��+3NH4+
��4��Mg ��5��a c d
������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ 2Al2O3(����)
 4Al+3O2��
4Al+3O2��Al3++3NH3��H2O=Al(OH)3��+3NH4+
��4��Mg ��5��a c d
����Ԫ�ص�λ�ÿ�֪��a��H��B��He��c��N��d��O��e��Mg��f��Al��g��S��h��Cl��
��1���������δ�ɶԵ���������Ԫ����N����3����
��2�����������ǽ��������Զ��ߵ��ں��������������������ǽ������塣
��3��ԭ�Ӻ�p�Dz��������չ����Ԫ��ΪAl�������������������������Ի����������Ϊ���Ǽ�����ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ�����ǻ��õĽ�����Ӧ��ͨ����ⷨ������ʽΪ2Al2O3(����) 4Al+3O2�����������������Ի�������������ڰ�ˮ�У�����ʽΪ
4Al+3O2�����������������Ի�������������ڰ�ˮ�У�����ʽΪ
Al3++3NH3��H2O=Al(OH)3��+3NH4+��
��4��þ�ܺ�������Ӧ��������þ��Ҳ�ܺ͵�����Ӧ���ɵ���þ��
��5����������ᡢ�����������������Ҫ��Ԫ����H��N��O��
��1���������δ�ɶԵ���������Ԫ����N����3����
��2�����������ǽ��������Զ��ߵ��ں��������������������ǽ������塣
��3��ԭ�Ӻ�p�Dz��������չ����Ԫ��ΪAl�������������������������Ի����������Ϊ���Ǽ�����ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ�����ǻ��õĽ�����Ӧ��ͨ����ⷨ������ʽΪ2Al2O3(����)
 4Al+3O2�����������������Ի�������������ڰ�ˮ�У�����ʽΪ
4Al+3O2�����������������Ի�������������ڰ�ˮ�У�����ʽΪAl3++3NH3��H2O=Al(OH)3��+3NH4+��
��4��þ�ܺ�������Ӧ��������þ��Ҳ�ܺ͵�����Ӧ���ɵ���þ��
��5����������ᡢ�����������������Ҫ��Ԫ����H��N��O��

��ϰ��ϵ�д�
�����Ŀ
 ����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�����������������֮����
����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�����������������֮����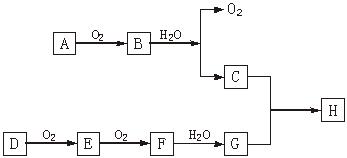
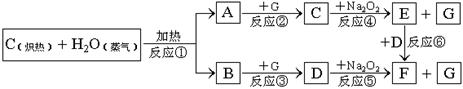
 ��E
��E  ����NA��ʾ����ӵ�������
����NA��ʾ����ӵ������� ol��
ol��
