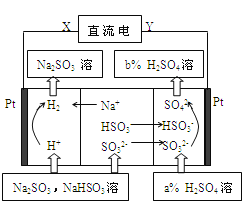
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩάϊ”Ο»γΆΦΥυ ΨΉΑ÷ΟΘ§ΦΉ÷– Δ”–100mL 0.1mol/LCuSO4»ή“ΚΘ§““÷– Δ”–100mL 0.2mol/L Na2SO4»ή“ΚΓΘ
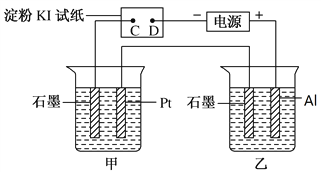
Θ®1Θ©Ά®Βγ“ΜΕΈ ±ΦδΘ§Ιέ≤λΒΫ Σ»σΒΡΒμΖέKI ‘÷ΫΒΡ_____(―ΓΧνΓΑCΓ±ΜρΓΑDΓ±)ΕΥ±δάΕΓΘ
Θ®2Θ©ΉΑ÷Ο““÷–Ιέ≤λΒΫΒΡœ÷œσ «_________________________________________ΓΘ
Θ®3Θ©»τΒγΫβ“ΜΕΈ ±ΦδΚσΘ§ΉΑ÷ΟΦΉΓΔ““÷–Ι≤ ’Φ·ΒΫΤχΧε0.168 L(±ξΉΦΉ¥Ωωœ¬)Θ§‘ρΘΚ
ΔΌΉΑ÷ΟΦΉ÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣ______________________________ΓΘ
ΔΎ»τΒγΫβ«ΑΚσΧεΜΐ±δΜ·Κω¬‘≤ΜΦΤΘ§‘ρΒγΚσΉΑ÷ΟΦΉ÷–»ή“ΚΒΡc(H+)ΈΣ________ΓΘ
Θ®4Θ©»τΒγΫβ“ΜΕΈ ±ΦδΚσΘ§ΉΑ÷ΟΦΉ÷–»ή“Κ–ηΦ”»κ0.005molΦν ΫΧΦΥαΆ≠≤≈ΡήΜ÷Η¥‘≠ά¥ΒΡ≈®Ε»ΚΆpHΘ§‘ρΒγΫβΙΐ≥Χ÷–ΉΣ“ΤΒΡΒγΉ” ΐΡΩΈΣ__________ΓΘ
Θ®5Θ©»τΉΑ÷Ο÷–ΒΡΒγ‘¥ Ι”Ο»γ”“ΆΦΒΡ“Μ÷÷–¬–Ά»ΦΝœΒγ≥ΊΘ§“‘ΦΉ¥ΦΈΣ»ΦΝœΘ§“ΜΕ®±»άΐΒΡLi2CO3ΚΆNa2CO3ΒΡ»έ»ΎΜλΚœΈοΈΣΒγΫβ÷ Θ§‘ρΗΚΦΪΖ¥”Π ΫΈΣ__________________ΓΘ
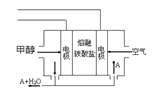
Θ®6Θ©ΒγΫβ“ΜΕΈ ±ΦδΚσΘ§≤πΫβΉΑ÷ΟΘ§ΫΪΆΦ÷–ΦΉΉΑ÷Ο»Γ≥ωΚσΘ§”ΟΒΦœΏΫΪ ·ΡΪΚΆPtΝ§Ϋ”ΙΙ≥…±’ΚœΜΊ¬ΖΓΘ‘ρ¥ΥΉΑ÷Ο÷–Pt…œΖΔ…ζΒΡΒγΦΪΖ¥”Π Ϋ «_____ΓΘ
ΓΨ¥πΑΗΓΩ C ·ΡΪΦΪ”–Έό…ΪΤχ≈ί≤ζ…ζΘ§¬ΝΒγΦΪ»ήΫβΘ§»ή“Κ÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμ 2Cu2++2H2O ![]() 2Cu+O2Γϋ+4H+ 0Θ°1mol/L 0Θ°03NA CH3OHΘ≠6eΘ≠ΘΪ3CO
2Cu+O2Γϋ+4H+ 0Θ°1mol/L 0Θ°03NA CH3OHΘ≠6eΘ≠ΘΪ3CO![]() ===4CO2ΘΪ2H2O O2+ 4H++ 4e- = 2H2O
===4CO2ΘΪ2H2O O2+ 4H++ 4e- = 2H2O
ΓΨΫβΈωΓΩΘ®1Θ©DΕΥ”κΒγ‘¥ΗΚΦΪœύΝ§ΈΣ“θΦΪΘ§CΕΥΈΣ―τΦΪΘ§―τΦΪ…œΒβάκΉ” ßΒγΉ”…ζ≥…ΒΞ÷ ΒβΘ§ΒΞ÷ Ββ”ωΒΫΒμΖέ»ή“ΚΜα±δάΕ…ΪΘ§Υυ“‘CΕΥ±δάΕ…ΪΘΜΘ®3Θ©““ΉΑ÷Ο÷–Al”κΒγ‘¥’ΐΦΪœύΝ§Ής―τΦΪΘ§ ßΒγΉ”…ζ≥…¬ΝάκΉ”Θ§ ·ΡΪΒγΦΪ «“θΦΪΘ§«βάκΉ”ΒΟΒγΉ”…ζ≥…«βΤχΚΆ«β―θΗυάκΉ”Θ§«β―θΗυάκΉ””κ¬ΝάκΉ”ΫαΚœ…ζ≥…«β―θΜ·¬ΝΑΉ…Ϊ≥ΝΒμΘ§Υυ“‘ Β―ιœ÷œσ « ·ΡΪΦΪ”–Έό…ΪΤχ≈ί≤ζ…ζΘ§¬ΝΒγΦΪ»ήΫβΘ§»ή“Κ÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμΘΜΘ®3Θ©ΔΌΉΑ÷ΟΦΉ÷–Pt”κ’ΐΦΪœύΝ§ΈΣ―τΦΪΘ§ ·ΡΪΈΣ“θΦΪΘ§“θΦΪ…œΆ≠άκΉ”ΒΟΒγΉ”…ζ≥…CuΘ§―τΦΪ…œ«β―θΗυ ß»ΞΒγΉ”…ζ≥…―θΤχΘ§Υυ“‘ΉήΒΡΖ¥”ΠΖΫ≥Χ ΫΈΣ2Cu2++2H2O![]() 2Cu+O2Γϋ+4H+ΘΜΔΎΦΉΓΔ““ΉΑ÷Ο÷–Ι≤ ’Φ·ΒΫ±ξΉΦΉ¥Ωωœ¬ΒΡΤχΧε0.168LΘ§Έο÷ ΒΡΝΩ «0.168LΓ¬22.4L/molΘΫ0.0075molΘ§Ζ÷±πΈΣ―θΤχΚΆ«βΤχΘ§‘ρ―θΤχΈΣ0.0075molΓΝ1/3=0.0025molΘ§ΗυΨίΒγΫβΒΡΉήΖ¥”Π ΫΩ…÷Σ»ή“Κ÷–«βάκΉ”ΒΡΈο÷ ΒΡΝΩ «0.0025molΓΝ4ΘΫ0.01molΘ§≈®Ε» «0.01molΓ¬0.1LΘΫ0.1mol/LΘΜΘ®4Θ©Φ”»κ0.005molΦν ΫΧΦΥαΆ≠[Cu2(OH)2CO3]œύΒ±”ΎΦ”»κ0.01molCuOΓΔ0.005molH2OΘ§ΗυΨί…ζ≥…Έο÷ΣΘ§“θΦΪ…œΆ≠άκΉ”ΚΆ«βάκΉ”Ζ≈ΒγΓΔ―τΦΪ…œ«β―θΗυάκΉ”Ζ≈ΒγΘ§ΗυΨίCu‘≠Ή”ΓΔH‘≠Ή” ΊΚψΒΟ“θΦΪ…œΈω≥ωn(Cu)ΘΫ0.01molΓΔn(H2)ΘΫ0.005molΘ§‘ρΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΘΫ0.01molΓΝ2+0.005molΓΝ2ΘΫ0.03molΘ§ ΐΡΩΈΣ0.03NAΘΜΘ®5Θ©‘≠Βγ≥ΊΒΡΗΚΦΪΖΔ…ζ ß»ΞΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΘ§‘ρΦΉ¥Φ‘ΎΗΚΦΪΖ≈ΒγΘ§”…”Ύ «»έ»ΎΒΡΧΦΥα―ΈΘ§“ρ¥ΥΗΚΦΪΖ¥”Π ΫΈΣCH3OHΘ≠6eΘ≠ΘΪ3CO32Θ≠ΘΫ4CO2ΘΪ2H2OΓΘΘ®6Θ©ΒγΫβ“ΜΕΈ ±ΦδΚσΘ§≤πΫβΉΑ÷ΟΘ§ΫΪΆΦ÷–ΦΉΉΑ÷Ο»Γ≥ωΚσΘ§”ΟΒΦœΏΫΪ ·ΡΪΚΆPtΝ§Ϋ”ΙΙ≥…±’ΚœΜΊ¬ΖΘ§ ·ΡΪΒγΦΪ”–ΗΫΉ≈ΒΡΆ≠Θ§“ρ¥Υ¥Υ ±ΙΙ≥…‘≠Βγ≥ΊΘ§ ·ΡΪΒγΦΪ «ΗΚΦΪΘ§PtΒγΦΪ «’ΐΦΪΘ§―θΤχ‘Ύ’ΐΦΪΖ≈ΒγΘ§”…”ΎΒγΫβ÷ »ή“Κœ‘Υα–‘Θ§‘ρΉΑ÷Ο÷–Pt…œΖΔ…ζΒΡΒγΦΪΖ¥”Π Ϋ «O2+ 4H++ 4eΘ≠ΘΫ2H2OΓΘ
2Cu+O2Γϋ+4H+ΘΜΔΎΦΉΓΔ““ΉΑ÷Ο÷–Ι≤ ’Φ·ΒΫ±ξΉΦΉ¥Ωωœ¬ΒΡΤχΧε0.168LΘ§Έο÷ ΒΡΝΩ «0.168LΓ¬22.4L/molΘΫ0.0075molΘ§Ζ÷±πΈΣ―θΤχΚΆ«βΤχΘ§‘ρ―θΤχΈΣ0.0075molΓΝ1/3=0.0025molΘ§ΗυΨίΒγΫβΒΡΉήΖ¥”Π ΫΩ…÷Σ»ή“Κ÷–«βάκΉ”ΒΡΈο÷ ΒΡΝΩ «0.0025molΓΝ4ΘΫ0.01molΘ§≈®Ε» «0.01molΓ¬0.1LΘΫ0.1mol/LΘΜΘ®4Θ©Φ”»κ0.005molΦν ΫΧΦΥαΆ≠[Cu2(OH)2CO3]œύΒ±”ΎΦ”»κ0.01molCuOΓΔ0.005molH2OΘ§ΗυΨί…ζ≥…Έο÷ΣΘ§“θΦΪ…œΆ≠άκΉ”ΚΆ«βάκΉ”Ζ≈ΒγΓΔ―τΦΪ…œ«β―θΗυάκΉ”Ζ≈ΒγΘ§ΗυΨίCu‘≠Ή”ΓΔH‘≠Ή” ΊΚψΒΟ“θΦΪ…œΈω≥ωn(Cu)ΘΫ0.01molΓΔn(H2)ΘΫ0.005molΘ§‘ρΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΘΫ0.01molΓΝ2+0.005molΓΝ2ΘΫ0.03molΘ§ ΐΡΩΈΣ0.03NAΘΜΘ®5Θ©‘≠Βγ≥ΊΒΡΗΚΦΪΖΔ…ζ ß»ΞΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΘ§‘ρΦΉ¥Φ‘ΎΗΚΦΪΖ≈ΒγΘ§”…”Ύ «»έ»ΎΒΡΧΦΥα―ΈΘ§“ρ¥ΥΗΚΦΪΖ¥”Π ΫΈΣCH3OHΘ≠6eΘ≠ΘΪ3CO32Θ≠ΘΫ4CO2ΘΪ2H2OΓΘΘ®6Θ©ΒγΫβ“ΜΕΈ ±ΦδΚσΘ§≤πΫβΉΑ÷ΟΘ§ΫΪΆΦ÷–ΦΉΉΑ÷Ο»Γ≥ωΚσΘ§”ΟΒΦœΏΫΪ ·ΡΪΚΆPtΝ§Ϋ”ΙΙ≥…±’ΚœΜΊ¬ΖΘ§ ·ΡΪΒγΦΪ”–ΗΫΉ≈ΒΡΆ≠Θ§“ρ¥Υ¥Υ ±ΙΙ≥…‘≠Βγ≥ΊΘ§ ·ΡΪΒγΦΪ «ΗΚΦΪΘ§PtΒγΦΪ «’ΐΦΪΘ§―θΤχ‘Ύ’ΐΦΪΖ≈ΒγΘ§”…”ΎΒγΫβ÷ »ή“Κœ‘Υα–‘Θ§‘ρΉΑ÷Ο÷–Pt…œΖΔ…ζΒΡΒγΦΪΖ¥”Π Ϋ «O2+ 4H++ 4eΘ≠ΘΫ2H2OΓΘ

ΓΨΧβΡΩΓΩΡ≥Ά§―ß…ηΦΤΝΥ»γœ¬ΧΫΨΩ Β―ιΖΫΑΗΘΚ
Β―ι | ≤ίΥα»ή“Κ (0.5mol/L) | ΗΏΟΧΥαΦΊ (0.5mol/L) | œΓΝρΥα (0.5mol/L) | ΝρΥαΟΧ (0.5mol/L) | Έ¬Ε» | ’τΝσΥ° |
ΔΌ | 10.0 mL | 2.0 mL | 3.0 mL | 0 | 25Γφ | 1.0 mL |
ΔΎ | 10.0 mL | 2.0 mL | 3.0 mL | 1.0 mL | 25Γφ | 0 |
Δέ | 8.0 mL | 2.0 mL | 3.0 mL | 0 | 25Γφ | Vx |
Δή | 10.0 mL | 2.0 mL | 3.0 mL | 0 | 35Γφ | 1.0 mL |
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©±ΨΖΫΑΗ÷–”Ο”Ύ…ηΦΤ Β―ιΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ «____________________Θ§ Β―ι÷–‘ΛΆ®ΙΐΙέ≤λ ≤Ο¥œ÷œσΜρ≤βΕ® ≤Ο¥ ΐΨίά¥Ϋχ––≈–ΕœΘΚ_____________________ΓΘ
Θ®2Θ©ΗΟ Β―ιΒΡΡΩΒΡ «________________________________________________ΓΘ
Θ®3Θ© Β―ιΔέ÷–ΒΡVx ΘΫ______ΘΜ Β―ιΔΌΚΆΔή≤βΒΟΖ¥”ΠΥΌ¬ Ζ÷±πΈΣv1ΓΔv4Θ§‘ρv1_____v4(ΧνΘΚΓΑΘΨΓ±ΓΑΘΦΓ±ΚΆΓΑΘΫΓ±)ΓΘ
Θ®4Θ© Β―ιΔΌ÷–Θ§c(Mn2+)”κtΙΊœΒ»γ”“ΆΦΥυ ΨΓΘABΕΈ–±¬ Οςœ‘¥σ”ΎOAΕΈ–±¬ Θ§≥ΐΖ¥”ΠΩ…ΡήΖ≈»»ΆβΘ§ΜΙΩ…Ρή «________________ΓΘ