��Ŀ����
��10�֣�A��B��C��D��E���ֿ����Ի�����ֱ���������Fe3+��Ba2+��Al3+��Na+��Ag+��������NO3����OH����SO42����Cl����CO32���еĸ�һ����ɣ����Ӳ��ظ�������������ʵ�飺
��A��E����Һ�Լ��ԣ�0.1mol��L��1A��Һ��pHС��13��
����B����Һ����μ��백ˮ�а�ɫ�������ɣ������Ӱ�ˮ��������������ʧ��
����C����Һ�м������ۣ���Һ���������ӣ�
����D����Һ�м��������Ba(OH)2��Һ��û�г�����
��ش��������⣺
��1������������ʵ�ƶ�A��E�Ļ�ѧʽ��
A ��B ��C ��D ��E ��
��2���������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� ��
��3��д���ۡ��ܵ����ӷ���ʽ���� ���� ��
��A��E����Һ�Լ��ԣ�0.1mol��L��1A��Һ��pHС��13��
����B����Һ����μ��백ˮ�а�ɫ�������ɣ������Ӱ�ˮ��������������ʧ��
����C����Һ�м������ۣ���Һ���������ӣ�
����D����Һ�м��������Ba(OH)2��Һ��û�г�����
��ش��������⣺
��1������������ʵ�ƶ�A��E�Ļ�ѧʽ��
A ��B ��C ��D ��E ��
��2���������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� ��
��3��д���ۡ��ܵ����ӷ���ʽ���� ���� ��
��1��A��Na2CO3 B��AgNO3 C��Fe2(SO4)3 D��AlCl3 E��Ba(OH)2 (��1�֣�
��2��CO32����H2O HCO3����OH�� ��2�֣�
HCO3����OH�� ��2�֣�
��3����Fe��2Fe3+��3Fe2+ ��2�֣� ��Al3+��4OH����AlO2����2H2O ��2�֣�
��2��CO32����H2O
 HCO3����OH�� ��2�֣�
HCO3����OH�� ��2�֣���3����Fe��2Fe3+��3Fe2+ ��2�֣� ��Al3+��4OH����AlO2����2H2O ��2�֣�
��1����B����Һ����μ��백ˮ�а�ɫ�������ɣ������Ӱ�ˮ��������������ʧ������B������������C����Һ�м������ۣ���Һ���������ӣ���C�к��������ӣ���D����Һ�м��������Ba(OH)2��Һ��û�г�����˵��D��û��SO42��������C����������A��E����Һ�Լ��ԣ�0.1mol��L��1A��Һ��pHС��13������A��̼���ƣ���E������������D���Ȼ�����
��2��̼������ǿ�������Σ�ˮ���Լ��ԣ�����ʽ��CO32����H2O HCO3����OH����
HCO3����OH����
��3��д���ۡ��ܵ����ӷ���ʽ�ֱ���Fe��2Fe3+��3Fe2+��Al3+��4OH����AlO2����2H2O��
��2��̼������ǿ�������Σ�ˮ���Լ��ԣ�����ʽ��CO32����H2O
 HCO3����OH����
HCO3����OH������3��д���ۡ��ܵ����ӷ���ʽ�ֱ���Fe��2Fe3+��3Fe2+��Al3+��4OH����AlO2����2H2O��

��ϰ��ϵ�д�
�����Ŀ
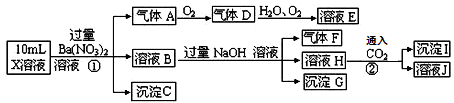
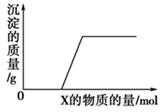
 ��NO3����OH��
��NO3����OH�� \x(����B����HClð���̰�ɫ����C
\x(����B����HClð���̰�ɫ����C