��Ŀ����
����Ŀ������к͵ζ�����ѧ��ѧ����������һ����Ҫ��������֪��25��ʱ��ȡ20.00mLŨ�Ⱦ�Ϊ0.1000mol/L��������HX��HY��HZ����Ũ��Ϊ0.1000mol/L��NaOH��Һ�ֱ���еζ����ζ�������ͼ��ʾ������˵������ȷ����( )��

A.����ͬ�¶��£�ͬŨ�ȵ���������Һ��pH��С˳��Ϊ��HZ<HY<HX
B.��V(NaOH)=10mLʱ����HY��Һ����ˮ�������![]()
C.HY��HZ��ϣ��ﵽƽ��ʱ��![]()
D.���������ζ����ߣ��ɼ����Ka(HY)��10-3
���𰸡�D
��������
A����ͼ��֪δ�μ�NaOH��Һʱ��ͬŨ�ȵ���������ҺpH��С˳��ΪHZ<HY<HX����A��ȷ��
B����V(NaOH)=10mLʱ����Һ������Ϊ�����ʵ�����HY��NaY����Һ��pH=5��������Һ��c(H+)=10-5mol/L������Һ�е�c(OH��)=10-9mol/L���������ʿ�֪������ȫ����ˮ���룬��ˮ�������������Ũ�Ⱥ���������ͬ������ˮ�������c(H+)=10-9mol/L����B��ȷ��
C��HY��HZ��ϣ���Һ�е���غ�Ϊ��c(H+)=c(Y��)+c(Z��)+c(OH��)������c(H+)-c(Y��)=c(Z��)+c(OH��)����C��ȷ��
D����ͼ��֪0.1000mol/L��HY��ҺpH=3��c(H+)=10-3 mol/L��Ka(HY)=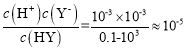 ����D����
����D����
�ʴ�ΪD��

����Ŀ���ο�����ͼ�����й�Ҫ��ش����⣺
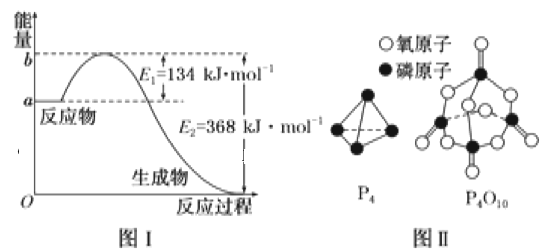
��1��ͼ����1molNO2(g)��1molCO(g)��Ӧ����CO2��NO�����������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯��__(�������С�����䡱����ͬ)����H�ı仯��__����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��__��
��2���±��Dz��ֻ�ѧ���ļ������ݣ�
��ѧ�� | P��P | P��O | O=O | P=O |
����(kJ��mol-1) | 198 | 360 | 498 | x |
��֪1mol����(P4)��ȫȼ�շ���Ϊ1194kJ����������ȫȼ�յIJ���ṹ��ͼ����ʾ�������x=__kJ��mol-1��
��3��PCl5��һ����Ҫ�ĺ���������л��ϳ��������Ȼ�����ij�¶�ʱ����2.0L���º����ܱ������г���1.0molPCl5��������ӦPCl5(g)![]() PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3��/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
��Ӧ��ǰ50s��ƽ������v(PCl5)=__���ڸ��¶��£�����ʼʱ����0.5molPCl5��amolCl2��ƽ��ʱPCl5��ת������Ϊ20������a=__��