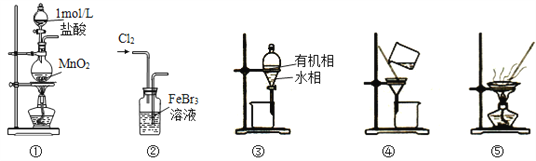
��Ŀ����
����Ŀ��������(CH3CH2CH2CH2OCH2CH2CH2CH3)��һ�ֻ���ԭ�ϣ�������Ϊ��ɫҺ�壬������ˮ���е�Ϊ142.4�棬�ܶȱ�ˮС��ijʵ��С����������װ�úϳ������ѣ�����װ�þ���ȥ������������Ҫ��ӦΪ��
![]()
ʵ��������£����ݻ�Ϊl00mL��������ƿ�н�5mLŨ���ᡢ14.8g�������ͼ�����ʯ��Ͼ��ȣ��ټ��Ȼ���һ��ʱ�䣬�ռ����ֲ�Ʒ�����Ƶõ������ѡ��ش��������⣺

��1���ϳɴֲ�Ʒʱ��Һ���Լ�����˳����_________________��
��2��ʵ��������ˮӦ��____���������a����b������
��3��Ϊ��֤��Ӧ�¶Ⱥ㶨��135��C��װ��C����ʢҺ�������е���������Ϊ________��
��4������ʱ��������¶ȹ��ߣ���Ӧ���Һ���ڣ�д����ŨNaOH��Һ�����ж�β�������ӷ���ʽ________________��
��5���õ��������Ѵֲ�Ʒ������8 mL50%�����ᡢ10 mLˮ��ȡϴ�ӡ��ò�������Ҫ�����ڹ����β��ʵ�ʵ���������ձ�����������________________________��
��6����ʵ�����յõ�6.50g�����ѣ��������ѵIJ�����________________________��
���𰸡� �ȼ������������Ũ���� a�� ��Һ��е����135�� 2OH-+SO2��SO32-+H2O�� ��Һ©�� 50.0%
����������1��Ũ����������Һ����ʱ���ȼ�����Һ�������ᣬ��ֹŨ����ϡ��ʱ���ȣ�����Һ��ɽ�������Һ���Լ�����˳�����ȼ������������Ũ���ᣬ�ʴ�Ϊ���ȼ������������Ũ���ᡣ
��2��������������ʱ������ˮ�����������������෴��������ˮ��a�ڽ��룬�ʴ�Ϊ��a��
��3������Һ���ܴﵽ������¶ȵ�����е㣬��Ϊ��֤��Ӧ�¶Ⱥ㶨��135�棬װ��C����ʢҺ��ķе�Ӧ�ô���135�棬�ʴ�Ϊ����Һ��е����135����
��4������ʱ��������¶ȹ��ߣ���Ӧ���Һ���ڣ�Ũ�����붡������������ԭ��Ӧ��Ũ���ᱻ��ԭ���ɶ�������������������Һ���ն�������������������������Ƶķ�Ӧ���ӷ���ʽΪ��2OH-+SO2�TSO32-+H2O���ʴ�Ϊ��2OH-+SO2�TSO32-+H2O��
��5������ֲ��Һ���÷�Һ©�����ò�������Ҫ�����ڹ����β��ʵ�ʵ���������ձ�������������Һ©�����ʴ�Ϊ����Һ©����
��6����7����![]()
74��2 130
14.8g mg
��m=��14.8g��130������74��2��=13.0 g������=(6.5 g��13.0 g )��100%=50.0% ���ʴ�Ϊ��50.0%��

����Ŀ����������ʵ����ʵ�Ľ��ͣ�����������
ѡ�� | ʵ����ʵ | ���� |
A | ��������MgSO4��Һ�ܵõ�MgSO4���壻��������MgCl2��Һ�ò���MgCl2���� | H2SO4���ӷ���HCl�ӷ� |
B | ���CuCl2��Һ�������õ�Cu�����NaCl��Һ�������ò���Na | �õ���������Cu2+>Na+>H+ |
C | ŨHNO3������NO��ϡHNO3��������NO | HNO3Ũ��Խ��������Խǿ |
D | �����Ҵ���Ӧƽ��������ˮ��Ӧ���� | �ǻ�����Ļ��ԣ�C2H5OH2O |
A. A B. B C. C D. D
����Ŀ���ױ���һ�������£����������ױ��Ļ����ͱ����й����ʵķе㡢�۵����£�
�Զ��ױ� | �ڶ��ױ� | ����ױ� | �� | |
�е�/�� |
|
|
|
|
�۵�/�� |
|
|
|
|
����˵������ȷ����
A. �ױ����ɶ��ױ������ͱ��ķ�Ӧ����ȡ����Ӧ
B. �ױ��ķе����![]() ��
��
C. ������ķ����ɽ����ӷ�Ӧ���ò��������ȷ������
D. �Ӷ��ױ�������У�����ȴ�ᾧ�ķ����ɽ��Զ��ױ��������