��Ŀ����
������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ��֪ʶ�ش��±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
�Իش��������⣺
��1����25��t1��t2�����________1��10��14���������������������
��2��25��ʱ��ijHCl��Һ��c(HCl)=1��10��4 mol��L��1�������Һ��pH�� ����ʱc��H+��H2O�� mol/L����ˮϡ��1000������ϡ�ͺ���Һ��pH____ _7���������������������
��3��t2��ʱ����pH��11��������������ҺV1 L��pH��1��ϡ����V2 L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH��2����V1��V2=____________��
| �¶� / �� | 25 | t1 | t2 |
| ˮ�����ӻ����� | 1��10��14 | �� | 1��10��12 |
�Իش��������⣺
��1����25��t1��t2�����________1��10��14���������������������
��2��25��ʱ��ijHCl��Һ��c(HCl)=1��10��4 mol��L��1�������Һ��pH�� ����ʱc��H+��H2O�� mol/L����ˮϡ��1000������ϡ�ͺ���Һ��pH____ _7���������������������
��3��t2��ʱ����pH��11��������������ҺV1 L��pH��1��ϡ����V2 L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH��2����V1��V2=____________��
(1)�� (2)4��1��10��10 ���� (3)9:11(2��)
�����������1��ˮ��������ʣ����ڵ���ƽ�⡣�����������ȵģ����ȴٽ�ˮ�ĵ��룬˭�����ӻ����������������25��t1��t2�������1��10��14��
��2��25��ʱ��ijHCl��Һ��c(HCl)��1��10��4 mol��L��1������Ԫ����ǿ�ᣬ��ȫ���룬��˸���Һ��������Ũ����1��10��4 mol��L��1��������Һ��pH��4����ʱc��H+��H2O������Һ��OH��Ũ�ȣ�������
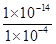 ��1��10��10mol/L����ˮϡ��1000������������Խ��ͣ����������Լ��ԣ�������ϡ�ͺ���Һ��pH��7��
��1��10��10mol/L����ˮϡ��1000������������Խ��ͣ����������Լ��ԣ�������ϡ�ͺ���Һ��pH��7����3��t2��ʱ��ˮ�����ӻ�������1��10��12����ʱpH��11��������������Һ��Ũ����0.1mol/L��V1 L����Һ��pH��1��ϡ����V2 L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH��2����˵����Ӧ�������ǹ����ģ���
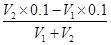 ��0.01����V1��V2��9:11��
��0.01����V1��V2��9:11��
��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 H++OH�����ƣ�����Һ�����Ե���
H++OH�����ƣ�����Һ�����Ե���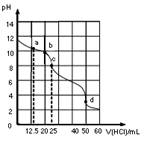
 ��
�� ��
�� ��NaOH ��
��NaOH �� ��
�� �����и���������ȷ����
�����и���������ȷ���� ����>��>��>��
����>��>��>�� : ��>��>��>��
: ��>��>��>��