��Ŀ����
��A,B,C,D,E���ֶ�����Ԫ��,A��B���γ�BA�ͻ�����,��AԪ���Ƿǽ�������ǿ��Ԫ��.����B��ԭ�Ӻ�������������ǰһ����ͬ����Ԫ��ԭ�ӵ���������8��;CԪ��������ͬλ��C1,C2,C3,��Ȼ���ﺬ������C1,C3����������C1��3��,C2��ԭ�ӵ���������C1��2��.D����̬�⻯���ˮ��Һ�ʼ���,��������������ˮ����Ϊǿ��,EԪ��ԭ�ӵ������������ȴ�����������4��,E���ӵĺ������������������2��.
(1)д��Ԫ������:A. B. C. D . E. .
(2)д��C1,C2, C3���ӵķ���: , ,
(3)д��E���ӵĽṹʾ��ͼ E���⻯��ķ���ʽ .
(4)д��A�ĵ�����B�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ ;
(5)д��A�ĵ��ʺ�B�ĵ��ʷֱ���ˮ��Ӧ�Ļ�ѧ����ʽ , ,�����߷�Ӧ�����ɵ���Һ���,������Ӧ�����ӷ���ʽΪ
(6)A��C�γɵĻ������к���ѧ�������� .�õ���ʽ��ʾ�û�������γɹ��� .
(1)д��Ԫ������:A. B. C. D . E. .
(2)д��C1,C2, C3���ӵķ���: , ,
(3)д��E���ӵĽṹʾ��ͼ E���⻯��ķ���ʽ .
(4)д��A�ĵ�����B�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ ;
(5)д��A�ĵ��ʺ�B�ĵ��ʷֱ���ˮ��Ӧ�Ļ�ѧ����ʽ , ,�����߷�Ӧ�����ɵ���Һ���,������Ӧ�����ӷ���ʽΪ
(6)A��C�γɵĻ������к���ѧ�������� .�õ���ʽ��ʾ�û�������γɹ��� .
��ÿ�ո�1�֣���16�֣� (1)��;�ƣ��⣻������ (2) 1 1H,2 1H(D),3 1H(T)
��3�� �� H2O ��4��2Na+F2=2NaF
�� H2O ��4��2Na+F2=2NaF
��5��2F2+2H2O=4HF+O2����Na+2H2O=2NaOH+H2���� OH-+HF=F-+H2O
(6) ���ۼ�
���ۼ�
��3��
 �� H2O ��4��2Na+F2=2NaF
�� H2O ��4��2Na+F2=2NaF��5��2F2+2H2O=4HF+O2����Na+2H2O=2NaOH+H2���� OH-+HF=F-+H2O
(6)
 ���ۼ�
���ۼ� ���������AԪ���Ƿǽ�������ǿ��Ԫ�أ���A��FԪ�ء�����B��ԭ�Ӻ�������������ǰһ����ͬ����Ԫ��ԭ�ӵ���������8�������Ը��ݻ�ѧʽBA��֪��B�ǵ�IAԪ�أ�����B��Na��CԪ��������ͬλ��C1,C2,C3,��Ȼ���ﺬ������C1,C3����������C1��3��,C2��ԭ�ӵ���������C1��2�����ɴ˿�֪��C����Ԫ�ء�D����̬�⻯���ˮ��Һ�ʼ��ԣ���������ˮ�Լ��ԣ�����D�ǵ�Ԫ�ء�EԪ��ԭ�ӵ������������ȴ�����������4��,E���ӵĺ������������������2������˵��E�Ƿǽ���Ԫ����Ԫ�ء�
�������������е��Ѷȵ����⣬�����ۺ���ǿ�������߿��������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

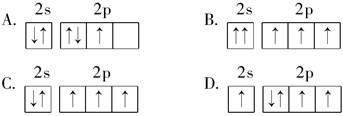

 Ϊ
Ϊ
 Һ������������ϡ����İ�ɫ��������B�Ļ�ѧʽ�� ��
Һ������������ϡ����İ�ɫ��������B�Ļ�ѧʽ�� ��