��Ŀ����
����Ŀ���������(K2FeO4)��һ��������ˮ��������25�棬��ˮ��Һ�м�����ı���Һ��pHʱ���������ӵ����ʵ���������(X)��pH�ı仯��ͼ��ʾ[��֪![]() ]������˵����ȷ���ǣ� ��
]������˵����ȷ���ǣ� ��
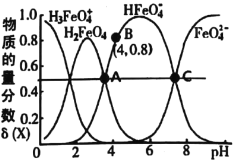
A.K2FeO4��H2FeO4������ǿ�����
B.��B�����ݿ�֪��H2FeO4�ĵ�һ�����볣��Ka1=4.0��10-4
C.25�棬H2FeO4(aq)+H+![]() H3FeO4+(aq)��ƽ�ⳣ��K��100
H3FeO4+(aq)��ƽ�ⳣ��K��100
D.A��C�����Ӧ��Һ��ˮ�ĵ���̶Ȳ����
���𰸡�BD
��������
A. ��Һ�д���HFeO4-��H2FeO4������H2FeO4����������ʣ���A����
B. ��B�����ݿ�֪��H2FeO4�ĵ�һ�����볣��Ka1= =4.0��10-4����B��ȷ��
=4.0��10-4����B��ȷ��
C. H2FeO4(aq)��H3FeO4+(aq)���ʱpHԼ����1.5��25����H2FeO4(aq)+H+![]() H3FeO4+(aq)��ƽ�ⳣ��K=
H3FeO4+(aq)��ƽ�ⳣ��K=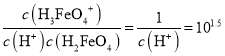 <100����C����
<100����C����
D. A�����Һ�����ԣ�˵��H2FeO4�������HFeO4-ˮ�⣬����ˮ���룬C����Һ�ʼ��ԣ�˵��HFeO4-����С��FeO42-ˮ�⣬�ٽ�ˮ���룬��Ӧ��Һ��ˮ�ĵ���̶Ȳ���ȣ���D��ȷ��
ѡBD��

����Ŀ������(![]() )����Ҫ�Ļ���ԭ�ϡ�ij��ȤС����ʵ��������ȡ������������
)����Ҫ�Ļ���ԭ�ϡ�ij��ȤС����ʵ��������ȡ������������
��֪����![]() ��NH3���ƣ������ᷴӦ����
��NH3���ƣ������ᷴӦ����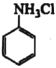 (������ˮ����)��
(������ˮ����)��
������������ȡ�����ķ�ӦΪ��2![]() +3Sn+12HCl
+3Sn+12HCl![]() 2
2![]() +3SnCl4+4H2O
+3SnCl4+4H2O
���й����ʵIJ����������ʼ��±���
���� | ��Է������� | �۵�/�� | �е�/�� | �ܽ��� | �ܶ�/g��cm-3 |
���� | 93 | 6.3 | 184 | ����ˮ������������ | 1.02 |
������ | 123 | 5.7 | 210.9 | ������ˮ������������ | 1.23 |
���� | 74 | 116.2 | 34.6 | ����ˮ | 0.7134 |
��.�Ʊ�����
ͼ1��ʾװ���м���20mLŨ����(����)��������ˮԡ�л���20min��ʹ��������ֻ�ԭ����ȴ����������ƿ�е���һ����50%NaOH��Һ������Һ�ʼ��ԡ�
��1���μ�����NaOH��Һ��Ŀ����___��д����Ҫ��Ӧ�����ӷ���ʽ___��
��.ȡ��ͼl��ʾװ���е�������ƿ����װΪͼ2��ʾװ�á�����װ��A����ˮ����������ˮ�����������ķ�����B�б�������������ƿC���ռ���������ˮ�Ļ�����������õ��ֱ�����ˮ��Һ�ס�
��.������ˮ��Һ���м����Ȼ��ƹ��������ͣ�����������ȡ���õ�������ȡҺ��
iii.�ϲ��ֱ�����������ȡҺ����NaOH�����������õ�����1.86g��
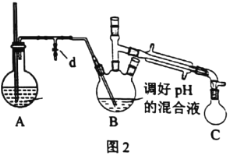
��2��װ��A�в����ܵ�������__��
��3���ڱ���������Ϻ�Ӧ���еIJ�������__����___��
��4����ʵ���б����IJ���Ϊ___(������λ��Ч����)��
��5�����ڲ����������³�ȥ���������������������ʣ�����ʵ�鷽��___��