��Ŀ����
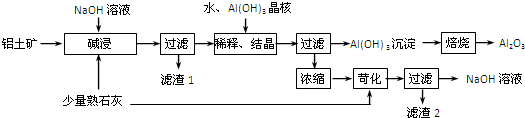
A��B��C��D��E����ѧ���������ֻ��������֮��������ת����ϵ��

��֪A��Һ�Լ��ԣ��ɴٽ�ˮ�ĵ��룻D��Һ�����ԣ�A��D��Һ��ɫ��Ӧ��Ϊ��ɫ��
��1����A��B��C�к�����ͬ�Ľ���Ԫ�أ���B��������Ҳ�����ڼD����Ba(OH)2��Ӧ���ɲ���������İ�ɫ��������A�Ļ�ѧʽΪ ��D�Ļ�ѧʽΪ ��A��C��Ӧ����B�����ӷ���ʽΪ ��
��2����C��һ����ɫ��ζ�����壬����ʹ����ʯ��ˮ����ǡ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� �� C��E��Ӧ����B�Ļ�ѧ����ʽΪ ��
��ϰ��ϵ�д�
�����Ŀ
7���谢���ӵ³���ΪNA��������˵����ȷ���ǣ�������
| A�� | 28g CO�к��еķ�����ΪNA | |
| B�� | ��״���£�22.4 L H2O�����ķ�����ΪNA | |
| C�� | ��״���£�22.4L H2������Ϊ1 g | |
| D�� | 1mol/L MgCl2��Һ�к�Mg2+��ΪNA |
8����������������������ǣ�������
| A�� | ������O3�� | B�� | ����� | C�� | �Ȼ��� | D�� | ����þ |
10��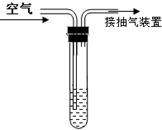 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��
Ϊ�ⶨ�����ж�������ĺ�����ij��ѧ����С���ͬѧ����SO2+I2+2H2O=H2SO4+2HI���������ͼ��ʾװ�ý���ʵ�飮
��1�������⣨I2��1.27mg�ĵ���Һ���뵽�Թ��У�
��2�����Թ��ڵμ�2��3�ε�����Һ����Һ����ɫ��������I2������
��3��ͨ������װ�ó�����ʹ�����ɵ����ܽ����Թ������Һ�Ӵ���
��4������Һ����ɫ��Ϊ��ɫʱ���ⶨͨ������������Ϊ1m3����ͨ�������жϣ��õ��������ж��������Ũ�ȼ���
 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��| ���� | һ��ָ�� | ����ָ�� | ����ָ�� |
| Ũ����ֵ��mg/m3�� | 0.15 | 0.50 | 0.70 |
��1�������⣨I2��1.27mg�ĵ���Һ���뵽�Թ��У�
��2�����Թ��ڵμ�2��3�ε�����Һ����Һ����ɫ��������I2������
��3��ͨ������װ�ó�����ʹ�����ɵ����ܽ����Թ������Һ�Ӵ���
��4������Һ����ɫ��Ϊ��ɫʱ���ⶨͨ������������Ϊ1m3����ͨ�������жϣ��õ��������ж��������Ũ�ȼ���
6�������������Ʒֱ�Ͷ������������������Ϊ100g��98%��HCl��H2SO4��H3PO4��Һ�У���������ȫ�μ��˷�Ӧ�������ɵ������ڱ�״���µ�������ǣ�������
| A�� | 1��2��3 | B�� | 3��2��1 | C�� | 1��1��1 | D�� | ������ |


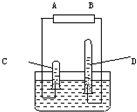 �����ӵ��������ܽ��һ����Ҫ���ɣ�ͬ��ͬѹ�£�ͬ������κ����庬����ͬ��Ŀ�ķ��ӣ���ͼ�ǵ��ˮ�ļ���װ�ã�
�����ӵ��������ܽ��һ����Ҫ���ɣ�ͬ��ͬѹ�£�ͬ������κ����庬����ͬ��Ŀ�ķ��ӣ���ͼ�ǵ��ˮ�ļ���װ�ã� ȫ�ܽ⣬������Һ��������0.56L����(STP)��ʹ���е�Fe2+ȫ��ת��ΪFe3+�����������Ļ�ѧʽΪ( )
ȫ�ܽ⣬������Һ��������0.56L����(STP)��ʹ���е�Fe2+ȫ��ת��ΪFe3+�����������Ļ�ѧʽΪ( )