��Ŀ����
(15��)�Ͼɼ���п�̸ɵ���ڲ��ĺ�ɫ����A��Ҫ����MnO2��NH4Cl��ZnCl2������������FeCl2��̿�ۣ���A�Ʊ��ߴ�MnCO3������ͼ���¡�
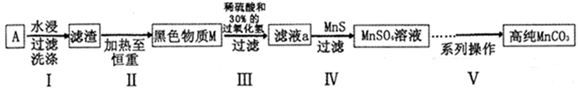
��1������п�̸ɵ�صĸ��������� (�ѧʽ)��
��2�� ��I�������������ijɷ��� ���ڢ�����Ŀ���� ��
��3����������Ƶ�MnSO4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪ ��
(��֪��Ksp(MnS)=2.5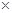 10-13,Ksp(ZnS)=1.6
10-13,Ksp(ZnS)=1.6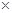 10-24)
10-24)
��5����֪��MnCO3������ˮ���Ҵ�����ʪʱ�ױ�����������l00oCʱ��ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpHΪ7.7��
��V��ϵ�в����ɰ����²�����У�
����l�������Լ�X������pH<7.7�� ����2�����ˣ�������ˮϴ��2~3�Σ�
����3�������Һ�� ����4����������ˮ�Ҵ�ϴ��2~3�Σ�
����5�����º�ɡ�
���Լ�X�� ��
�ڲ���3�У�˵��SO42-�ѳ��ɾ��ķ����� ��

��1������п�̸ɵ�صĸ��������� (�ѧʽ)��
��2�� ��I�������������ijɷ��� ���ڢ�����Ŀ���� ��
��3����������Ƶ�MnSO4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪ ��
(��֪��Ksp(MnS)=2.5
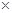 10-13,Ksp(ZnS)=1.6
10-13,Ksp(ZnS)=1.6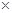 10-24)
10-24)��5����֪��MnCO3������ˮ���Ҵ�����ʪʱ�ױ�����������l00oCʱ��ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpHΪ7.7��
��V��ϵ�в����ɰ����²�����У�
����l�������Լ�X������pH<7.7�� ����2�����ˣ�������ˮϴ��2~3�Σ�
����3�������Һ�� ����4����������ˮ�Ҵ�ϴ��2~3�Σ�
����5�����º�ɡ�
���Լ�X�� ��
�ڲ���3�У�˵��SO42-�ѳ��ɾ��ķ����� ��
��1��Zn����2��MnO2��̿�ۣ���ȥ̿�ۡ���3��MnO2+H2O2+H2SO4 MnSO4+2O2+H2O��
MnSO4+2O2+H2O��
��4��MnS+Zn2+ ZnS+Mn2+����5����Na2CO3����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���
ZnS+Mn2+����5����Na2CO3����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���
 MnSO4+2O2+H2O��
MnSO4+2O2+H2O����4��MnS+Zn2+
 ZnS+Mn2+����5����Na2CO3����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���
ZnS+Mn2+����5����Na2CO3����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���������������������Ϣ��������֪����ɫ����A��Ҫ����MnO2��NH4Cl��ZnCl2������������FeCl2��̿�ۣ�MnO2��̿�۲�����ˮ����ˮ�������ˡ�ϴ�ӵ������ijɷ���MnO2��̿�ۣ�MnO2��̿�ۼ��������أ�̼��������е�������Ӧ����̼�����������ȥ����ɫ����M����Ҫ�ɷ�ΪMnO2��MnO2�����ᡢ˫��ˮ��Ӧ���������̡�������ˮ������ϵ�в����ĸߴ�MnCO3����1�����ݽ̲�֪ʶ֪������п�̸ɵ�صĸ���������Zn����2�� ����������֪����I�������������ijɷ���MnO2��̿�ۣ��ڢ�����Ŀ���dz�ȥ̿�ۡ���3��MnO2�����ᡢ˫��ˮ��Ӧ���������̡�������ˮ���÷�Ӧ�Ļ�ѧ����ʽΪMnO2+H2O2+H2SO4
 MnSO4+2O2+H2O����4����Ksp(MnS)=2.5
MnSO4+2O2+H2O����4����Ksp(MnS)=2.5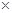 10-13,Ksp(ZnS)=1.6
10-13,Ksp(ZnS)=1.6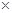 10-24֪��ZnS��MnS�����ܣ����ó�����ת����ȥZn2+�����ӷ���ʽΪMnS+Zn2+
10-24֪��ZnS��MnS�����ܣ����ó�����ת����ȥZn2+�����ӷ���ʽΪMnS+Zn2+ ZnS+Mn2+�� ��5����V��ϵ�в������������Ƶøߴ�MnCO3�����Լ�X��Na2CO3���ڲ���3Ϊ��������Ƿ�ϴ�Ӹɾ���˵��SO42-�ѳ��ɾ��ķ�����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���
ZnS+Mn2+�� ��5����V��ϵ�в������������Ƶøߴ�MnCO3�����Լ�X��Na2CO3���ڲ���3Ϊ��������Ƿ�ϴ�Ӹɾ���˵��SO42-�ѳ��ɾ��ķ�����ȡ���һ��ϴ��Һ���������Թ��У��������ᱵ�����ް�ɫ�������ɣ�˵��SO42-�ѳ��ɾ���
��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ

 HCO3����H2O
HCO3����H2O
 CO(g) ��H2(g) ��H=" +131.3" kJ?mol-1�����Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ�ӿ췴Ӧ���������������H2O��ƽ��ת���ʵ��� ��(�����)
CO(g) ��H2(g) ��H=" +131.3" kJ?mol-1�����Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ�ӿ췴Ӧ���������������H2O��ƽ��ת���ʵ��� ��(�����) CO2(g)��H2(g)���õ��������ݣ�
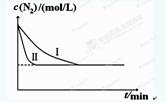
CO2(g)��H2(g)���õ��������ݣ� 2NH3(g) ��H��-92.4kJ?mol-1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯����ͼ��
2NH3(g) ��H��-92.4kJ?mol-1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯����ͼ��