��Ŀ����
���ᣨ��ѧʽ��HOCN����һ���лӷ��Ժ�ʴ�Ե�Һ�壬��ˮ�����̷������·�Ӧ�γ���X������̼泥���HOCN + 2H2O �� X��
(1). ��������Ӧ���漰�ĸ�Ԫ���У��뾶����Ԫ��ԭ�������ڱ��е�λ����___________����ԭ�Ӻ�����ӹ�ռ��_______�������
(2). X���������ӵĵ���ʽ��________����֪25��ʱ0.1mol/L X��ҺpH =7.8�������ӷ���ʽ��ʾԭ��______________________��
(3). ��˵����Ԫ�صķǽ����Ա�̼Ԫ��ǿ����ʵ��_________��ѡ���ţ���
a. ���õ��Ӷ�ƫ��H-N��H-C b. �����ԣ�NO2��CO2
c. ���ԣ�HNO3��H2CO3 d. �е㣺NH3��CH4
(4). �ݲⶨ�����������ֽṹ��һ�ַ����ں�����������Ϊ�����ᣬ��һ�ַ����ڲ�����������Ϊ�����ᣬ�����ֽṹ������ԭ���������Ѵﵽ�ȶ��ṹ��������Ҳ������״�ṹ����ֱ�д���������������Ľṹʽ��_______________��______________��
(1). ��������Ӧ���漰�ĸ�Ԫ���У��뾶����Ԫ��ԭ�������ڱ��е�λ����___________����ԭ�Ӻ�����ӹ�ռ��_______�������
(2). X���������ӵĵ���ʽ��________����֪25��ʱ0.1mol/L X��ҺpH =7.8�������ӷ���ʽ��ʾԭ��______________________��
(3). ��˵����Ԫ�صķǽ����Ա�̼Ԫ��ǿ����ʵ��_________��ѡ���ţ���
a. ���õ��Ӷ�ƫ��H-N��H-C b. �����ԣ�NO2��CO2
c. ���ԣ�HNO3��H2CO3 d. �е㣺NH3��CH4
(4). �ݲⶨ�����������ֽṹ��һ�ַ����ں�����������Ϊ�����ᣬ��һ�ַ����ڲ�����������Ϊ�����ᣬ�����ֽṹ������ԭ���������Ѵﵽ�ȶ��ṹ��������Ҳ������״�ṹ����ֱ�д���������������Ľṹʽ��_______________��______________��
.(1)�ڶ����ڢ�A 4 (2) NH4+ 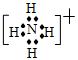 HCO3- +H2O
HCO3- +H2O H2CO3+ OH-
H2CO3+ OH-
(3) a c (4)H-O-C��N (2��) , O=C=N-H (2��)
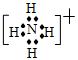 HCO3- +H2O
HCO3- +H2O H2CO3+ OH-
H2CO3+ OH-(3) a c (4)H-O-C��N (2��) , O=C=N-H (2��)
��1��HOCN + 2H2O �� NH4HCO3����Cԭ�ӵİ뾶��������ռ����1s2s2px2py�ĸ������
��2��NH4HCO3��Һ�ʼ��ԣ���Ҫ��HCO3-ˮ��ʼ��Զ����¡�
��3���жϷǽ�����ǿ�����䵥����������ϵ������Լ��⻯����ȶ��ԣ���ۺ����������ǿ��������֮��Ļ����û��ȡ������aѡ��ɿ���NԪ���������ӵ�����ǿ��C����ѡac��
��4�����ݸ�ԭ�Ӵﵽ�������軯ѧ���ĸ�������д��
��2��NH4HCO3��Һ�ʼ��ԣ���Ҫ��HCO3-ˮ��ʼ��Զ����¡�
��3���жϷǽ�����ǿ�����䵥����������ϵ������Լ��⻯����ȶ��ԣ���ۺ����������ǿ��������֮��Ļ����û��ȡ������aѡ��ɿ���NԪ���������ӵ�����ǿ��C����ѡac��
��4�����ݸ�ԭ�Ӵﵽ�������軯ѧ���ĸ�������д��

��ϰ��ϵ�д�
�����Ŀ
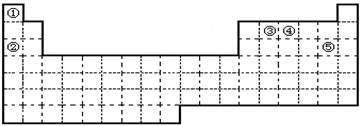
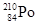 ��
��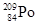 ��Ϊͬ��������
��Ϊͬ��������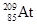 ��Ϊͬλ��
��Ϊͬλ��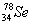 ��
��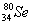 ��˵����ȷ���ǣ� ��
��˵����ȷ���ǣ� �� ��
��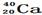
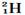 ��
��