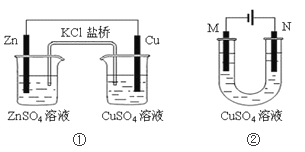
��Ŀ����
����Ŀ�����������һ����ֲ���͡������ͻ������֬��Ϊԭ�ϼӹ����ɵ����ȼ������Ҫ�ɷ�Ϊ��֬���������һ���ò����͵�Ϊԭ���Ʊ�������͵�ʵ�鲽�����£�
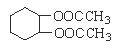
������ͼ��ʾ��������ƿ�м���3.2 g CH3OH��0.2 g NaOH���ܽ�������м���20 g(0.021��0.023 mol)�����ͼ�60 mL�����顣

��������60��65 �棬����2.5��3 h�����á��ϲ�Ϊ������͡������鼰�״����²���ҪΪ���͡�
����Һ��ˮϴ��
������������120��ʱ����Һ��������ʣ����Ϊ������͡�
��1��������п���n(CH3OH)/n(��֬)��3��Ŀ����________���������������________��
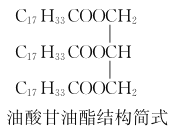
��2����������˵ļ��ȷ�ʽ��________�������͵ijɷ�֮һ���������(�ṹ��ʽ����ͼ)��״���Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
��3��������÷�Һ©������ʱ����ȡ�ϲ�Һ��ķ�����________����ˮϴ���ϲ�Һ��ʱ��˵����ϴ�Ӹɾ���������__________________________________��
���𰸡� ���������ʱ��֬��ת���� �ܼ� ˮԡ���� 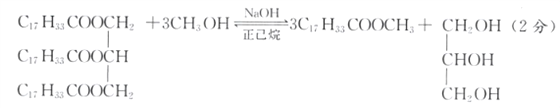 �ȷ�����²�Һ��Ȼ��رջ������ӷ�Һ©���Ͽڵ����ϲ�Һ�� ��pH��ֽ������ˮ�������
�ȷ�����²�Һ��Ȼ��رջ������ӷ�Һ©���Ͽڵ����ϲ�Һ�� ��pH��ֽ������ˮ�������
��������(1)�����������������Ҫ�ɷ�Ϊ��֬��������������͵���Ҫ�ɷ�Ϊ��֬��������������Լ���״���NaOH�������鹲ͬ�����£�������������Ӧ���������Ӧ��CH3OH�������������֬��ת���ʣ�������������Ϊ�ܼ����ܽ�����ʹٽ���Ӧ���У�
(2) �����¶�Ϊ60~65�����������˵ļ��ȷ�����ˮԡ�������������״���Ӧ�Ļ�ѧ����ʽΪ ��
��
(3) �÷�Һ©����Һʱ����ȡ�ϲ�Һ��ķ������ȷ�����²�Һ��Ȼ��رջ������ӷ�Һ©���Ͽڵ����ϲ�Һ�����ϲ�Һ��Ϊ������͡������鼰�״������ܺ���������NaOH��Һ����ˮϴ��ʱ������pH��ֽ���飬ˮ������ʱ��������ϴ�Ӹɾ���

����Ŀ���������Ρ����Ӽ������������HPF�����Һ�е���Ҫ��Ⱦ��(���л�����Cl����SO![]() ��Na����)������NaClO3��H2SO4���ѳ����Լ���
��Na����)������NaClO3��H2SO4���ѳ����Լ���
��1��Cl2���ȵ�NaOH��Һ��Ӧ����NaClO3���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
��2�� ����ʱ����ӦC6H5O����H2O ![]() C6H5OH��OH����ƽ�ⳣ��ΪK��________(C6H5OH��Ka��1��10��10)��
C6H5OH��OH����ƽ�ⳣ��ΪK��________(C6H5OH��Ka��1��10��10)��
��3�������÷�ˮ�辭Ԥ�������ٴ����Ρ�
����Ũ�����600 mL HPF�����Һ�����ữԤ����(��Ӧ�¶�100��)���������1��ʾ��
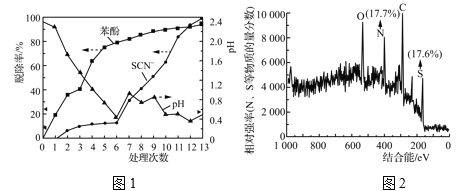
��1��Ԥ����ǰ����������Ũ�ȱ仯���
��Ŀ | S2O | SCN��(g��L��1) | ����(g��L��1) | pH |
����ǰ | 34.28 | 70.11 | 1.3 | 8.55 |
������ | 0.91 | 69.76 | 1.35 | 2.4 |
�ɱ�1��֪���ô�����������Ҫ����ȥ����Ⱦ����________��
����Ԥ�������Һ�����ٴ���(��Ӧ�¶�100 ��)ʱ��13���������Һ�м����Լ�������2��ʾ��
��2��13�����μ����Լ����
���� | 1 | 2 | 3 | 4 | span>5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
NaClO3/g | 16.3 | 0 | 0 | 0 | 0 | 0 | 7.6 | 0 | 5.7 | 0 | 10.9 | 0 | 7.1 |
ŨH2SO4/mL | 0 | 2 | 2 | 2 | 4 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 |
ʵ������ͼ1��ʾ��
ǰ![]() ��Cl����Ӧ����Cl2�йء��ð�ɫ�������ܵĽṹ��ʽΪ______________��
��Cl����Ӧ����Cl2�йء��ð�ɫ�������ܵĽṹ��ʽΪ______________��
���ɱ�2��ͼ1�����ݵó�����7��13�β�����SCN���ڽ�ǿ���������±�ClO![]() (��ˮ��)������������________���˹����в�������ש��ɫ����(����������ɫ����)���Գ�������XPS����������Ԫ�ص����ʵ���������ͼ2��ʾ����֪SCN���ɱ�ijЩ������(��Cu2����)��������Ϊש��ɫ�ij���(SCN)x��һ���Ʋ���Ϊ��ʵ���в�����ש��ɫ����Ϊ(SCN)x��֧�ָ��Ʋ��֤���У�________��
(��ˮ��)������������________���˹����в�������ש��ɫ����(����������ɫ����)���Գ�������XPS����������Ԫ�ص����ʵ���������ͼ2��ʾ����֪SCN���ɱ�ijЩ������(��Cu2����)��������Ϊש��ɫ�ij���(SCN)x��һ���Ʋ���Ϊ��ʵ���в�����ש��ɫ����Ϊ(SCN)x��֧�ָ��Ʋ��֤���У�________��
����Ŀ��ij��������Һ���������ӵ�Ũ�����±���ʾ����M����Ϊ��������
���� | NO3�� | SO42�� | H+ | M |
Ũ��/(mol/L) | 2 | 1 | 2 | 2 |
A.Cl��B.Ba2+C.Na+D.Mg2+