��Ŀ����
����Ŀ��ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ���
��1�����������У���õζ�ʵ���ص�Ϊ______��
A ��ͷ�ι� B ��Ͳ��10ml�� C ��ƿ D �ζ��ܼ�
��2����ȡһ������İ״����õ�����_______��������ʽ��������ʽ�����ζ���. ʵ����Ӧѡ��______��ָʾ����
��3���ζ������н������²������ֱ�ָ����������Եζ������Ӱ�죨����ƫ��������ƫ����������Ӱ������
��ζ�ǰδ�ñ�Һ��ϴ��ʽ�ζ���______��
��ζ�����ʱ���ӵζ��̶ܿ�______
�� ʢװ����Һ�ĵζ��ܣ��ζ�ǰ��������ݣ��ζ���������ʧ______
��4���ڵζ������У���c��CH3COO����< c��Na+��ʱ��������Һ��____������ţ���
A ���� B ���� C ���� D ��ȷ��
���𰸡�AB ��ʽ ��̪��Һ ƫ�� ƫ�� ƫ�� B
��������
�����к͵ζ�ԭ��ѡ��������������Һ�����ѡ��ζ��ܣ�����c (����) =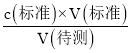 ��ʽ���з����ж����Ĵ�С��
��ʽ���з����ж����Ĵ�С��
(1) ����ʽ�ζ�����ȡ��һ������İ״�����ƿ�У����ݴ������������Ʒ�Ӧ�����˴����ƣ���Һ�ʼ��ԣ��ý�ͷ�ιܵμ�ָʾ�����ü�ʽ�ζ��ܶԴ�����еζ�����������к͵ζ�ʱ���ò�������������Ͳ��10mL������B��ȷ��
��2������ƿ����ȡһ������İ״����õ���������ʽ�ζ��ܣ���Ϊ�������������Ʒ�Ӧ�����˴����ƣ�����������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ������ѡ��̪;�𰸣���ʽ����̪��
��3��I�ζ�ǰδ�ñ�Һ��ϴ��ʽ�ζ��ܣ� ��ҺŨ�Ƚ��ͣ����V (��)ƫ����c (����) =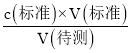 ������֪���ʴ�ΪC (����)ƫ��;
������֪���ʴ�ΪC (����)ƫ��;
II�ζ�����ʱ���ӵζ��̶ܿȣ����¶����ı�Һ���ƫС������c (����) = =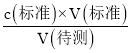 ������֪��C (����)ƫ�ͣ��ʴ�Ϊ:ƫ�ͣ�
������֪��C (����)ƫ�ͣ��ʴ�Ϊ:ƫ�ͣ�
�� ʢװ����Һ�ĵζ��ܣ��ζ�ǰ��������ݣ��ζ���������ʧ�����V (��)ƫ����c (����) =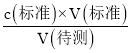 ������֪���ʴ�ΪC (����)ƫ��;
������֪���ʴ�ΪC (����)ƫ��;
��4���ڵζ�������,������Һ�гɷ����,������:c(CH3COO- ) +c(OH -) = c(Na+)+c(H+)����c��CH3COO����< c��Na+��ʱ, c(OH- )>c(H+) ,�����Һ�ʼ���;
����Bѡ������ȷ��;�𰸣�B��
(5)�ڵζ������У�������Һ�гɷ���� ����: c ( CH3COO-) +c (OH- )=c(Na+)+c(H+) ��
��c ( CH3COO-) <c ( Na+)ʱ��C(OH-)>c(H+) �����Һ�ʼ���;��B�������⣻

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�