��Ŀ����
����Ŀ����������������ij�ֶ����ڳ���Ԫ�أ�A��GΪ�ǽ������ʣ�A������Ϊ��ɫ���壬GΪ��������Ҫ�ɷ�֮һ��D������Ϊ��ɫҺ�壬C��E�����¾�Ϊ���壬E���γ������Ԫ�ף���Է���������G��2����F������Ϊ����ɫ���塣���ǵ�ת����ϵ��ͼ��ʾ��

��1��д����ѧʽ�� B________ �� F________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
�٣�_____________________________________________________________��
����_____________________________________������˫���ŷ������Ӧ���еĵ���ת�������
�ۣ�___________________________________________________________
���𰸡�H2SO4Na2O2C+2H2SO4(Ũ)=��=CO2��+2SO2��+2H2O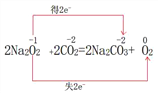
![]()
�������� ���⿼���й�Ԫ�ؼ��仯������ƶϼ����ʵ�֪ʶ�㣬�ɴ������������ɫ����ɡ���Ӧ�����ʵȷ������֣�����A������Ϊ��ɫ������Ϊ�ǽ������ʣ���֪AΪ̼���ʣ�D������Ϊ��ɫҺ�壬��֪DΪH2O��F������Ϊ����ɫ���壬������������G��G�ǿ�����Ҫ�ɷ�֮һ����֪FΪNa2O2,CΪCO2��GΪO2��E���γ������Ԫ�ף�����ΪSO2��NO2����Է���������O2��2��������Է�������Ϊ64��EΪΪSO2 ������A��̼����B����C��CO2����D��E��SO2����֪BΪH2SO4����DΪH2O��HΪSO3��
��1�����Ϸ�����֪��BΪH2SO4��FΪNa2O2
��2���ٷ���������ԭ��ӦΪ��C+2H2SO4(Ũ) CO2��+2SO2��+2H2O
�� ΪNa2O2��CO2��Ӧ��Na2O2�������������ǻ�ԭ����������Ԫ�ش�-1�����ߵ�O2�е�0�ۣ����͵�Na2CO3�е�-2�ۣ�ת��2�����ӣ�����ʽΪ��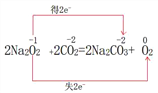
��ΪSO2�Ĵ�������Ӧ������ʽΪ��![]()



