��Ŀ����
���й��ھ����˵���У�����ȷ����
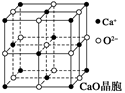
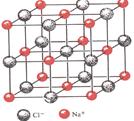
�پ�����ԭ�ӳ��������������У����Է��ԣ����Ǿ�����ԭ����������������Է��ԡ��ں��н��������ӵľ���һ�������Ӿ��塡�۹��ۼ��ɾ������Ӿ�����ۡ��е㡡��MgO�ľ�����Զ��NaCl��������Ϊǰ�����������ĵ�����࣬���Ӱ뾶С���ݾ����Ǿ���ṹ�Ļ�����Ԫ�������ڲ�������һ���������������ظ����С����御���ܲ�ȡ���ܶѻ���ʽ����ʹ���ñȽ��ȶ��߸ɱ������У������е�8��������������ľ���һ�����ӣ���һ��CO2������Χ��12��CO2���ӽ��ڣ����еķ��Ӿ��嶼�����ܶѻ�
�پ�����ԭ�ӳ��������������У����Է��ԣ����Ǿ�����ԭ����������������Է��ԡ��ں��н��������ӵľ���һ�������Ӿ��塡�۹��ۼ��ɾ������Ӿ�����ۡ��е㡡��MgO�ľ�����Զ��NaCl��������Ϊǰ�����������ĵ�����࣬���Ӱ뾶С���ݾ����Ǿ���ṹ�Ļ�����Ԫ�������ڲ�������һ���������������ظ����С����御���ܲ�ȡ���ܶѻ���ʽ����ʹ���ñȽ��ȶ��߸ɱ������У������е�8��������������ľ���һ�����ӣ���һ��CO2������Χ��12��CO2���ӽ��ڣ����еķ��Ӿ��嶼�����ܶѻ�
| A���٢ڢ� | B���ڢۢ� | C���ܢݢ� | D���ڢۢ� |
D
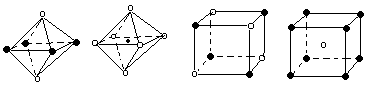
������������н��������ӵľ��岻һ�������Ӿ��壬�������������Ҳ���������ӣ��ڲ���ȷ���������Ӿ�����ۡ��е���Ƿ��Ӽ����������۲���ȷ�����еķ��Ӿ��岻һ���������ܶѻ�����������߲���ȷ������ѡ�����ȷ�ģ���ѡD��
�����������۷е�ߵͱȽϵ�һ������ǣ�ԭ�Ӿ��壬�۷е��С���ۼ���ǿ���й�ϵ�����������У��γɽ������Ľ��������Ӱ뾶ԽС�������Խ�࣬������Խǿ���۷е�Խ�ߣ����Ӿ������γɷ��Ӿ���ķ��Ӽ�������Խ���۷е�Խ�ߣ����Ӿ������γ����Ӽ������Ӱ뾶ԽС�������Խ�࣬���Ӽ�Խǿ���۷е�Խ�ߡ�

��ϰ��ϵ�д�
�����Ŀ
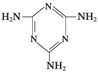 �׳ơ����������������������谷����������
�׳ơ����������������������谷����������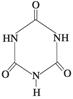 ��
��