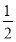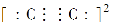ЬтФПФкШн
ЯТСаШмвКжагаЙиЮяжЪЕФСПХЈЖШЙиЯЕе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎNaHSO3ШмвКГЪЫсадЃЌдђгаЃКcЃЈNaЃЋЃЉЃОcЃЈHSO3-ЃЉЃОcЃЈSO32-ЃЉЃОcЃЈHЃЋЃЉЃОcЃЈOHЃЃЉ
BЃЎpHЯрЕШЕФCH3COONaКЭNa2CO3СНжжШмвКЃКcЃЈCH3COONaЃЉЃМcЃЈNa2CO3ЃЉ
CЃЎЧПЫсHAШмвКгыШѕМюMOHШмвКЛьКЯКѓШмвКГЪжаадЃЌдђгаЃКcЃЈMЃЋЃЉЃНcЃЈAЃЃЉ
DЃЎ0.1 molЁЄLЃ1ЕФNaHAШмвКpHЃН1ЃЌдђгаЃКcЃЈNaЃЋЃЉЃНcЃЈH2AЃЉЃЋcЃЈHAЃЃЉЃЋcЃЈA2ЃЃЉ
C
ЁОНтЮіЁПAЯюЃЌбЧСђЫсЧтИљРызгЕчРыЩњГЩЧтРызгКЭбЧСђЫсИљРызгЃЌЫЎДцдкЮЂШѕЕФЕчРыЩњГЩЧтРызгКЭЧтбѕИљРызгЃЌЫљвдЧтРызгХЈЖШДѓгкбЧСђЫсИљРызгХЈЖШЃЌAДэЃЛBЯюЃЌЯрЭЌХЈЖШЕФCH3COONaКЭNa2CO3ШмвКЃЌCH3COOЃЕФЫЎНтГЬЖШаЁгкCO32-ЕФЫЎНтГЬЖШЃЌЫљвдpHЯрЭЌЕФCH3COONaКЭNa2CO3ШмвКЃКcЃЈCH3COONaЃЉЃОcЃЈNa2CO3ЃЉЃЛCЯюЃЌИљОнЕчКЩЪиКуЕУcЃЈMЃЋЃЉЃЋcЃЈHЃЋЃЉЃНcЃЈAЃЃЉЃЋcЃЈOHЃЃЉЃЌгЩгкШмвКГЪжаадЃЌдђcЃЈHЃЋЃЉЃНcЃЈOHЃЃЉЃЌЫљвдcЃЈMЃЋЃЉЃНcЃЈAЃЃЉЃЛDЯюЃЌ0.1 molЁЄLЃ1ЕФNaHAШмвКpHЃН1ЃЌдђNaHA=NaЃЋЃЋHЃЋЃЋA2ЃЃЌЫљвдШмвКжаВЛДцдкHAЃКЭH2AЁЃ