��Ŀ����
ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
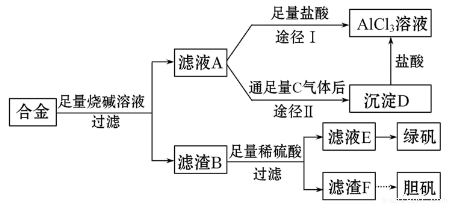
�Իش��������⣺
��1�������õ�����������ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������_______________��
��2������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ��������_____________��
��3������ҺE�еõ��̷������ʵ�������_______________________________��
��4��д��������F�Ʊ���������Ļ�ѧ����ʽ_____________________________��
��5����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ��������________��������___________��
��1��©����������
��2��;����
��3������Ũ������ȴ�ᾧ
��4��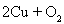
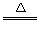
CuO+H2SO4=CuSO4+H2O��
CuSO4+5H2O=CuSO4��5H2O���𰸲�Ωһ���������ɣ�
��5�������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤
����������1���������װ�ó�����������֪�貹��IJ���������©���Ͳ���������2��������֪��ҺAΪNaAlO2��Һ������;����������ӦNaAlO2+4HCl=NaCl+AlCl3+2H2O������AlCl3��Һ�лẬ�д���NaCl���ʣ�����;����ʱ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3�����˵ô���Al��OH��3��Al��OH��3+3HCl=AlCl3+3H2O������AlCl3�������ʣ��ʺ�����;��Ϊ��3������BΪFe��Cu������ϡ���ᷴӦ��������ҺΪFeSO4������ΪCu������Һ����Ũ������ȴ�ᾧ���ɵ��̷�����4����Cu�Ʊ�����һ�㾭�����¼�����Ӧ��
2Cu+O2=2CuO��
CuO+H2SO4=CuSO4+H2O��
CuSO4+5H2O=CuSO4��5H2O��
��5������ͬѧ�ķ����辭���µ�ת����
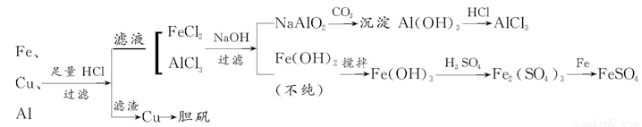
��Ȼ��������࣬�����Լ�����������ʱ�������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


