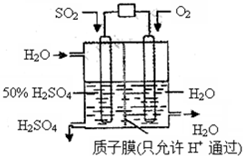
��Ŀ����
��ֹ�����ĸ�ʴ�����缶���⣬ÿ��ȫ����ֲ������ķ�֮һ��ʴ����ʧ��������ͼ�ش�

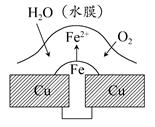
(1)������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ________________________��
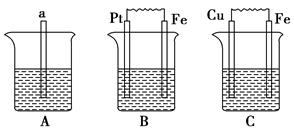
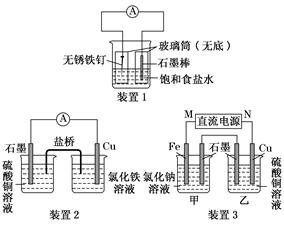
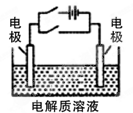
(2)Ϊ����ijˮ�����բ�ŵı���ʴ���ʣ����Բ���ͼ���������к�������բ���ϵĹ������R���Բ���__________��
(3)ͼ����ʾ����Ҳ�ɽ�����բ�ŵı���ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����

(1)������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ________________________��
(2)Ϊ����ijˮ�����բ�ŵı���ʴ���ʣ����Բ���ͼ���������к�������բ���ϵĹ������R���Բ���__________��
| A��ͭ | B���� | C��п | D��ʯī |
(1)������2Fe��4e��=2Fe2�� ������O2��4e����2H2O=4OH��
(2)C��(3)��
(2)C��(3)��
(1)����������ʴʱ��FeΪ������Fe��2e��=Fe2������ʴ�������ϣ�O2��4e����2H2O=4OH����
(2)��բ��������һ����������õĽ�����п�Ϳ���пʧȥ���ӱ���ʴ����Fe����������
(3)����ӵ�������������������ѱ����������(բ��)���Դ�ĸ��������ӡ�
(2)��բ��������һ����������õĽ�����п�Ϳ���пʧȥ���ӱ���ʴ����Fe����������
(3)����ӵ�������������������ѱ����������(բ��)���Դ�ĸ��������ӡ�

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ