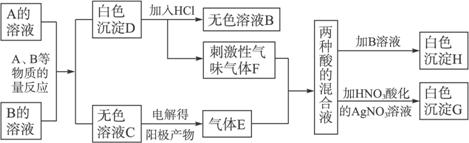
��Ŀ����
A��B��Ϊ��ɫ���壬����A����ɫ��Ӧ����ɫ(����ɫ�ܲ���)��A��ˮ��ҺpH����7��������A��B�����������е�ʵ���¼(����ͼ)������H��G��Ϊ��������İ�ɫ������
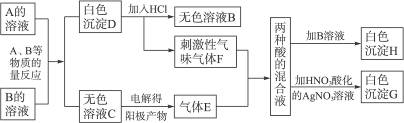
(1)д��A��B�����εĻ�ѧʽ��A________________��B________________��
(2)д��A��B��Ӧ�����ӷ���ʽ________________________��
(3)д��F+E+H2O��Ӧ�Ļ�ѧ����ʽ________________��
(4)���C����ɫ��Һ������������������������������ֵΪ1��1����ʵ���ռ���������������ȴ����1��1������ԭ��________________________��
(1)K2SO3 BaCl2
(2)![]() +Ba2+=====BaSO3��
+Ba2+=====BaSO3��
(3)SO2+Cl2+2H2O====![]() +2Cl-+4H+
+2Cl-+4H+
(4)��������Cl2���ܽ�ȴ�����������H2���ܽ��
����:
�̼�����ζ����R+����![]() E������Ļ��Һ���Բ²�ΪSO2+Cl2
E������Ļ��Һ���Բ²�ΪSO2+Cl2![]() H2SO4+ HCl����ȻHΪBaSO4(BΪBaCl2)��CΪAgCl��Ȼ������˼άDΪBaSO3��CΪKCl��
H2SO4+ HCl����ȻHΪBaSO4(BΪBaCl2)��CΪAgCl��Ȼ������˼άDΪBaSO3��CΪKCl��

��ϰ��ϵ�д�
�����Ŀ