��Ŀ����
����˵����ȷ����
- A.�ڳ����£����ȷ�Ӧһ�����Է����У����ȷ�Ӧ�������Է�����
- B.NH4HCO3(s)=====NH3(g)+H2O(g)+CO2(g)��H="+185.57" kJ��mol-1�����Է����У�ԭ
������ϵ���Է�������Ҷ����ӵķ���ת������� - C.��Ϊ�ʱ���ر䶼�뷴Ӧ���Է����йأ�����ʱ���ر�����Ե�����Ϊ��Ӧ��
���Ե��о� - D.����������������������£�ʹ�ô��������Ըı仯ѧ��Ӧ���еķ���
B
���������̼��ȼ��Ϊ���ȷ�Ӧ�������䲻���Է����У���ijЩ�ֽⷴӦ�ڳ�����ȴ���Խ��У���A���� NH4HCO3(s)=====NH3(g)+H2O(g)+CO2(g) ��H="+185.57" kJ��mol-1�����Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת�������C��ȷ��Ϊ����Ĵ𰸣��ʱ���ر�������Ե�����Ϊ��Ӧ�Է��Ե��оݣ���C��������������������������£�ʹ�ô����������Ըı仯ѧ��Ӧ���еķ���D����
���㣺��ѧ��Ӧ
���������⿼���˻�ѧ��Ӧ�����֪ʶ�������ڶԻ���֪ʶ�Ŀ��飬����Ҫ���յ����ʱ���ر�������Ե�����Ϊ��Ӧ�Է��Ե��оݣ�����ʲô���������������ܸ��ѧ��ƽ��㣬�����Ѷ����С�
���������̼��ȼ��Ϊ���ȷ�Ӧ�������䲻���Է����У���ijЩ�ֽⷴӦ�ڳ�����ȴ���Խ��У���A���� NH4HCO3(s)=====NH3(g)+H2O(g)+CO2(g) ��H="+185.57" kJ��mol-1�����Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת�������C��ȷ��Ϊ����Ĵ𰸣��ʱ���ر�������Ե�����Ϊ��Ӧ�Է��Ե��оݣ���C��������������������������£�ʹ�ô����������Ըı仯ѧ��Ӧ���еķ���D����
���㣺��ѧ��Ӧ
���������⿼���˻�ѧ��Ӧ�����֪ʶ�������ڶԻ���֪ʶ�Ŀ��飬����Ҫ���յ����ʱ���ر�������Ե�����Ϊ��Ӧ�Է��Ե��оݣ�����ʲô���������������ܸ��ѧ��ƽ��㣬�����Ѷ����С�

��ϰ��ϵ�д�
�����Ŀ
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ����о���Ӧ���ʵ��й����⣮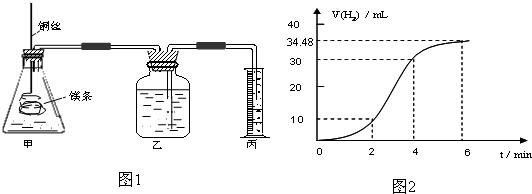
��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ����� ��������ţ�
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ ��������ͼ2����������ѻ���Ϊ��״���µ����������Һ����仯�ɺ��ԣ�
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
���ϱ���V1= mL��V3= mL��
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬�������� ��
����ʵ����t2��t3��t4����ɵó���ʵ������� ��

��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ�����
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬��������
����ʵ����t2��t3��t4����ɵó���ʵ�������


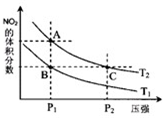 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�