��Ŀ����
��14�֣�����A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ����������������֪A��E��D��G�ֱ�ͬ���壻E��F��G��Hͬ���ڣ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N��B������������������Ӳ�����2����D�ǵؿ��к�������Ԫ�أ�Fλ��B��ǰһ���塣��ش��������⣺
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ���������ӷ���ʽ֤�� ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ���� �������ӷ���ʽ��ʾ����������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ���������ӷ���ʽ֤�� ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ���� �������ӷ���ʽ��ʾ����������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1����2���ڢ�A�壻 ������
��2��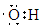 ��
��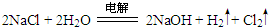
��3��Al(OH)3 + OH��==AlO2�� + 2H2O
��4��NH3��NH3 + H3O+ ="=" NH4+ + H2O
��5��S2��+ H2O HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
��2��
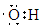 ��
��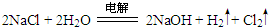
��3��Al(OH)3 + OH��==AlO2�� + 2H2O
��4��NH3��NH3 + H3O+ ="=" NH4+ + H2O
��5��S2��+ H2O
 HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol���������D�ǵؿ��к�������Ԫ����ΪO(��Ԫ��)��B������������������Ӳ�����2���������ΪHe��C��S�����ԭ������������BӦΪC(̼Ԫ��)��Fλ��B��ǰһ���壬��FΪAl(��Ԫ��)����D��Gͬ���壬��GΪS(��Ԫ��)�� E��F��G��Hͬ���ڣ�HΪCl(��Ԫ��)��A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N����AΪH(��Ԫ��)��CΪN(��Ԫ��)��MΪNH3��NΪH2O��A��Eͬ���壬��EΪNa��
��1��Ԫ��BΪ̼Ԫ�������ڱ���λ�ڵ�2���ڢ�A�壬MΪNH3���ռ乹���������Ρ�
��2��A��D��E����Ԫ�����һ�ֳ���������ΪNaOH���û������������ΪOH-�����������ͬ��ԭ���������Ŀ�Ҳ����������Ϊ�ǻ�(��OH)�������ʽΪ
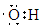 ����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ
����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ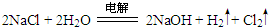 ��
����3��E��FԪ�ص�����������Ӧ��ˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ�����ӷ���ʽΪAl(OH)3 + OH��==AlO2�� + 2H2O
��4��MΪNH3��NΪH2O������NH3�����H+����Ϊ�ɷ�����ӦNH3 + H3O+ ="=" NH4+ + H2O��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y�ֱ�ΪNa2O2��Na2S������Na2S��ˮ��Һ�Լ��Ե�ԭ����������ˮ�⣬���ӷ���ʽΪS2��+ H2O
 HS�� + OH�� ��������7.8 g Na2O2���ʵ���Ϊ0.1mol����ˮ��Ӧ�ų�Q kJ������Q��0�����ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
HS�� + OH�� ��������7.8 g Na2O2���ʵ���Ϊ0.1mol����ˮ��Ӧ�ų�Q kJ������Q��0�����ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
��ϰ��ϵ�д�
�����Ŀ
 ��
�� �õ���������ͬ
�õ���������ͬ

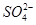 �Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
�Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________�� �����ӡ���֪
�����ӡ���֪ �Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________�� N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��
N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��