��Ŀ����
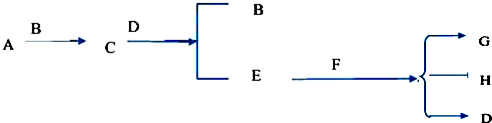
14����2L�ܱ������м���0.15mol•L-1A��0.05mol•L-1C��һ������B�������壮һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ�м�ͼ��ʾ[t0��t1ʱc��B��δ������t1ʱ����0.05mol•L-1]����ͼΪt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�������淴Ӧ������ʱ��仯�������
��1����t4ʱ�ı������Ϊ��Сѹǿ����B����ʼ���ʵ���Ϊ0.04mol��
��2����t5ʱ�ı�����������£���ʱv��������v���棩����A�����ʵ�������0.03molʱ�������������Ƚ�������Ϊa kJ��д����Ӧ���Ȼ�ѧ����ʽ��3A ��g��
 2C��g��+B��g����H=+100akJ/mol��
2C��g��+B��g����H=+100akJ/mol����3��t3ʱ�ı��ijһ��Ӧ����������CD��ѡ����ţ���
A��t3ʱ�̣�������X��Ũ�� B��t3ʱ�̣���������ϵ�¶�
C��t3ʱ�̣���С��������� D��t3ʱ�̣�ʹ���˴���
��4���ں��º�ѹ��ͨ��������壬v������=v���棩�����������=����������
��5����ͼ����������A��B�У�A�������ݻ����ֲ��䣬B�������ֺ�������ѹһ�£���ʼʱ���ڱ����������������ȵ�����£��ֱ�ͬʱ����2moLH2S��1moLSO2����Ӧ��ʼ���������ڷ�Ӧƽ����Ӧ����AС��B ������ڡ�����С�ڡ����ڡ�����
���� ��1����t4ʱ�ı������Ϊ��Сѹǿ��ƽ�ⲻ�ƶ���˵����Ӧǰ������������ȣ�ͼ����t1ʱ����ƽ�⣬��c��A��=0.09mol/L����c��C��=0.06mol/L������ѧ������֮��Ϊ0.09��0.06=3��2����BΪ�������Ӧ����ʽΪ��3A��g��?B��g��+2C��g��������B��Ũ�ȱ仯����B����ʼŨ��=B��ƽ��Ũ��-B��Ũ�ȱ仯�����ٸ���n=cV����B����ʼ���ʵ�����
��2����t5ʱ�ı�����������£���ʱv��������v���棩��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����A�����ʵ�������0.03molʱ�������������Ƚ�������Ϊa kJ����3mol A��Ӧ�ų������յ�����ΪakJ��$\frac{3mol}{0.03mol}$=100a kJ��ע�����ʾۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��3��t3 ʱ�ı�����˲���������ʶ�����ƽ�ⲻ�ƶ�����Ӧǰ������������䣬����������ѹǿ����ʹ�ô�����
��4���ں��º�ѹ��ͨ��������壬ƽ�ⲻ�����ƶ���
��5����ʼA��B������ѹǿ��ȣ�������Ӧ��2H2S+SO2=3S+H2O��AΪ���ݣ��淴Ӧ����ѹǿ��С����B��ѹǿ���䣬ѹǿԽ��Ӧ����Խ�죮
��� �⣺��1����t4ʱ�ı������Ϊ��Сѹǿ��ƽ�ⲻ�ƶ���˵����Ӧǰ������������ȣ�ͼ����t1ʱ����ƽ�⣬��c��A��=0.09mol/L����c��C��=0.06mol/L������ѧ������֮��Ϊ0.09��0.06=3��2����BΪ�������Ӧ����ʽΪ��3A��g��?B��g��+2C��g�������c��B��=$\frac{1}{2}$��c��C��=0.03mol/L��B����ʼŨ��=0.05mol/L-0.03mol/L=0.02mol/L����B����ʼ���ʵ���Ϊ2L��0.02mol/L=0.04mol��
�ʴ�Ϊ��0.04��
��2����t5ʱ�ı�����������£���ʱv��������v���棩��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����A�����ʵ�������0.03molʱ�������������Ƚ�������Ϊa kJ����3mol A��Ӧ�ų������յ�����ΪakJ��$\frac{3mol}{0.03mol}$=100a kJ����Ӧ�Ȼ�ѧ����ʽΪ��3A ��g��  2C��g��+B��g����H=+100akJ/mol��
2C��g��+B��g����H=+100akJ/mol��
�ʴ�Ϊ��3A ��g��  2C��g��+B��g����H=+100akJ/mol��
2C��g��+B��g����H=+100akJ/mol��
��3��t3 ʱ�ı�����˲���������ʶ�����ƽ�ⲻ�ƶ�����Ӧǰ������������䣬����������ѹǿ����ʹ�ô������ʴ�Ϊ��CD��
��4���ں��º�ѹ��ͨ��������壬ƽ�ⲻ�����ƶ�����v������=v���棩��
�ʴ�Ϊ��=��
��5����ʼA��B������ѹǿ��ȣ�������Ӧ��2H2S+SO2=3S+H2O��AΪ���ݣ��淴Ӧ����ѹǿ��С����B��ѹǿ���䣬ѹǿԽ��Ӧ����Խ�죬�ʷ�Ӧ��ʼ���������ڷ�Ӧƽ����Ӧ����A��B��
�ʴ�Ϊ��С�ڣ�
���� ���⿼�黯ѧƽ�������Ӱ�����ء���ѧƽ��ͼ��Ӧ���ʼ�����Ӱ�����ء��Ȼ�ѧ����ʽ��д�ȣ��ؼ��Ǹ���ͼ���ۺϷ���ȷ����Ӧ����ʽ���Ѷ��еȣ�

| A�� | C3H4��C3H6O | B�� | C3H6��C3H8O2 | C�� | C3H6O2��C3H8O | D�� | C3H8O��C4H6O |
��1���ϳɰ���ҵ�У��ϳ�����ÿ����1mol NH3���ų�46.1kJ������
�ٺϳɰ����Ȼ�ѧ����ʽ��N2��g��+3H2��g��=2NH3��g������H=-92.2KJ/mol��
��������ͬ������Ͷ��amol N2��bmol H2����ƽ��ʱ���ų�184.4kJ��������a�������������������=������2��
��2�������йغϳɰ���˵������ȷ����D��
A�������������Ϊ���ͷ�Ӧ�ġ�H����߷�Ӧ����
B���ڽϸ��¶��·�Ӧ����Ϊ��߷�Ӧ���ƽ��ת����
C���ʵ���ߵ���ȣ���Ϊ��߷�Ӧ������������ӿ췴Ӧ����
D��ѡ���ѹ����Ϊ�ӿ췴Ӧ���ʣ�����߷�Ӧ��ƽ��ת����
��3���ϳɰ�ԭ���е�H2���ü�����ˮ�����ڸ����·�Ӧ�Ƶã���֪����.1mol������ȫȼ�����ɳ������ȶ������������������±���
| ���� | ��H��kJ/mol�� |
| CO��g�� | -283.0 |
| CH4��g�� | -890.3 |
��.2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
��CH4ȼ��������̬������Ȼ�ѧ����ʽ��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802.3kJ/mol��
��CH4��ˮ�����ڸ����·�Ӧ�õ�CO��H2���Ȼ�ѧ����ʽ��CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.1kJ/mol��
| A�� | ij��Һ��NaOH ��Һ���ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬˵��ԭ��Һ�д���NH4+ | |
| B�� | ij��Һ�м���AgNO3 ��Һʱ��������ɫ������˵��ԭ��Һ��һ������Cl- | |
| C�� | ij��Һ�еμ�BaCl2 ��Һʱ��������ɫ������˵��ԭ��Һ��һ������SO42- | |
| D�� | ��ɫ��Ӧ�л����Ի�ɫ��˵����Ʒ�д�����Ԫ�أ������ڼ�Ԫ�� |
| A�� | 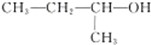 ������Ϊ2-��-1-���� ������Ϊ2-��-1-���� | |||||||||||||||||
| B�� | �������µ��Ų����ɣ���15�����ʿ���������Ʒ�Ӧ
| |||||||||||||||||
| C�� | 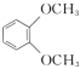 �ں˴Ź��������г�������壬����ԭ�Ӹ���֮��Ϊ3��2 �ں˴Ź��������г�������壬����ԭ�Ӹ���֮��Ϊ3��2 | |||||||||||||||||
| D�� | �ױ�����������ԭ�Ӿ���ͬһƽ�� |