��Ŀ����
����Ŀ��ʵ����������ͼ��ʾװ�����Ʊ���Ȳ������֤��Ȳ��ijЩ��ѧ���ʣ��Ʊ��� ��Ȳ�������������������� H2S ��PH3���壬�밴����Ҫ����գ�

(1)ʵ��������Ȳ�Ļ�ѧ����ʽ�ǣ�_________________��Ϊ�˵õ���Ϊƽ�ȵ���Ȳ������ װ�� A �ķ�Һ©���г���______________������ˮ��
(2)װ�� B ��CuSO4��Һ��������_______________________________��
(3)װ�� D �й۲쵽�������� ___________________________________________
(4)����ȡm g ��ʯ����Ӧ��ȫ�����ɵ���Ȳn g����CaC2�Ĵ���Ϊ_____(��m��n��ʾ)��
(5)д������Ȳ��HClΪԭ�ϣ��ϳɾ�����ϩ�ķ���ʽ��________________��___________________ ��
���𰸡�CaC2+2H2O��CH��CH��+Ca(OH)2 ����ʳ��ˮ ��ȥ H2S��PH3 ���ʣ��Է����ź���ʵ�� ��ɫ���Ϻ�ɫ��ȥ 32n/13m CH![]() CH + HCl
CH + HCl![]()
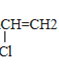

��������
�����л�������Ľṹ�����ʷ�����𣻸�����Ȳ���Ʊ�����������𣻸������ʵ��Ʊ������롢�ᴿ���֪ʶ�������
(1)ʵ�����Ʊ���Ȳ�����õ�ʯ��ˮ��Ӧ������Ȳ����������,��Ӧ�Ļ�ѧ����ʽΪ��CaC2+2H2O��CH��CH��+Ca(OH)2��̼������ˮ��Ӧ�ܾ��ң��ñ���ʳ��ˮ���Լ�����Ӧ������Ϊ�˵õ�ƽ�ȵ��������ñ���ʳ��ˮ����ˮ��
�ʴ�Ϊ�� CaC2+2H2O��CH��CH��+Ca(OH)2������ʳ��ˮ��
(2) ����������л�ԭ�ԣ�Ҳ�ᵼ����ˮ�����������Һ��ɫ��װ��B��CuSO4��Һ�������dz�ȥ��Ȳ�е������PH3��Cu2++H2S=CuS��+2H+����ֹ���ź�������ʵ�飻
�ʴ�Ϊ����ȥ H2S��PH3 ���ʣ��Է����ź���ʵ�飻
(3) ��Ȳͨ�����������Һ����Ȳ���в����ͼ��������������Һ�������ɶ�����̼��װ��D�й۲쵽����������Һ��ɫ�������ķ�ӦΪ������Ӧ��
�ʴ�Ϊ����ɫ��ȥ��
(4)n(C2H2)=![]() mol�����ݷ���ʽ�л�ѧ������ϵ��֪��n(CaC2)= n(C2H2)=
mol�����ݷ���ʽ�л�ѧ������ϵ��֪��n(CaC2)= n(C2H2)=![]() mol����m(CaC2)= n(CaC2)��64g/mol=
mol����m(CaC2)= n(CaC2)��64g/mol=![]() ��̼���ƵĴ���Ϊ��
��̼���ƵĴ���Ϊ��![]() =
=![]() =
=![]() ��
��
�ʴ�Ϊ��![]() ��
��
(5)��Ȳ��HCl�����ӳɷ�Ӧ�õ�����ϩ������ϩ��һ�������·����Ӿ۷�Ӧ���ɾ�����ϩ����ʽΪ��CH![]() CH+HCl��CH(Cl)=CH2��
CH+HCl��CH(Cl)=CH2�� ��
��
�ʴ�Ϊ��CH![]() CH+HCl��CH(Cl)=CH2��
CH+HCl��CH(Cl)=CH2�� ��
��

����Ŀ��������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ
���Ʊ�������ͻ���δ��Ӧ�ļױ�
��Ӧԭ����

��1����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ��
��2�������˷�Ӧ�����õ���Һ�����������õ��л����ˮ�㣻
��3�������л����м���ˮNa2SO4�����ˣ����������õ���ɫҺ��A��
��4������ˮ�����Ũ�����ữ������Ũ������ȴ�����ˣ��õ���ɫ����B��
��֪��
��Է������� | �۵� | �е� | �ܶ� | �ܽ�� | |
�ױ� | 92 | ��95�� | 110.8�� | 0.8669g��mL��1 | ������ˮ |
������ | 122 | 122.4�� | 249�� | 1.2659 g��mL��1 | 0.3g (25��ʱ) 6.9g (95��ʱ) |
(1)���������õIJ����������ձ���______________��������Ϊ________________��
(2)��3���м�����ˮNa2SO4��Ŀ����_____________________����ɫҺ��A��_______��
���ᴿ�ֱ�����
(3)��ͬѧ�����ؽᾧ�ķ����Եõ���B�����ᴿ���ؽᾧ�Ĺ��̣�__________��_________ ��_______ ������(���������)��ϴ�����������������ᡣ(ע������װ����ͼ��ʾ����Ҫ������A����©����B����ƿ�������õ�)

(4)��ɫ����B�е�������____________��
(5)���ȳ��˵õ�����Һ������ȴ���Խᾧ�������ı����ᾧ�壬Ϊ�˵õ�����ı����ᣬ�Dz����¶�Խ��Խ�ò�˵������______(������������������)������___________________________________________��