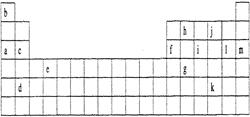
��Ŀ����
B��C��D��E���Ƕ�����Ԫ�ء�BԪ��ԭ��������ϵĵ�����Ϊ�ڲ����������2����BԪ��ԭ�Ӻ�CԪ��ԭ������������֮��Ϊ2��C��D�γɵĻ����������ɫ��Ӧ����ʻ�ɫ��EԪ�ص���̬���ʡ�E������������ˮ���������D������������ˮ���ﷴӦ��
(1)����4��Ԫ�ذ���ԭ�������ɴ�С����Ϊ____��____��____��____������Ԫ�ط��ţ���
(2) B��C�γɵĻ�����(�ȿ�����)�ĽṹʽΪ ��
(3)C��D�ĵ����ڼ�����������������X�����к��л�ѧ��������Ϊ_____��X�ĵ���ʽΪ ��
(4)Y��C����̬�⻯������£���D�ĵ���Ͷ��Y�У�������Ӧ�����ӷ���ʽΪ__________________���÷�Ӧ__________(��ܡ��������ܡ�)��Ϊ���ԭ��صķ�Ӧԭ����
(1)����4��Ԫ�ذ���ԭ�������ɴ�С����Ϊ____��____��____��____������Ԫ�ط��ţ���
(2) B��C�γɵĻ�����(�ȿ�����)�ĽṹʽΪ ��
(3)C��D�ĵ����ڼ�����������������X�����к��л�ѧ��������Ϊ_____��X�ĵ���ʽΪ ��
(4)Y��C����̬�⻯������£���D�ĵ���Ͷ��Y�У�������Ӧ�����ӷ���ʽΪ__________________���÷�Ӧ__________(��ܡ��������ܡ�)��Ϊ���ԭ��صķ�Ӧԭ����
(1)Cl��Na��O��C (2) 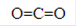
(3) ���Ӽ���(�Ǽ���)���ۼ�
(4) 2Na��2H2O 2 Na+��2 OH-��H2�� ��
2 Na+��2 OH-��H2�� ��
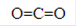
(3) ���Ӽ���(�Ǽ���)���ۼ�

(4) 2Na��2H2O
 2 Na+��2 OH-��H2�� ��
2 Na+��2 OH-��H2�� �������������������ƶϳ�B��C��D��E�ֱ�ΪC��O��Na��Cl������(1)����4��Ԫ�ذ���ԭ�������ɴ�С����ΪCl��Na��O��C��(2) B��C�γɵĻ�����ΪCO2,��ṹʽΪ
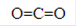 ��(3)C��D�ĵ����ڼ�����������������XΪNa2O2,�京�л�ѧ��������Ϊ���Ӽ���(�Ǽ���)���ۼ��������ʽΪ
��(3)C��D�ĵ����ڼ�����������������XΪNa2O2,�京�л�ѧ��������Ϊ���Ӽ���(�Ǽ���)���ۼ��������ʽΪ ��(4)YΪH2O,DΪNa,���Խ�D�ĵ���Ͷ��Y�У�������Ӧ�����ӷ���ʽΪ
��(4)YΪH2O,DΪNa,���Խ�D�ĵ���Ͷ��Y�У�������Ӧ�����ӷ���ʽΪ2Na��2H2O
 2 Na+��2 OH-��H2������Ϊ�÷�ӦΪ������ԭ��Ӧ�����Ը÷�Ӧ����Ϊ���ԭ��صķ�Ӧԭ����
2 Na+��2 OH-��H2������Ϊ�÷�ӦΪ������ԭ��Ӧ�����Ը÷�Ӧ����Ϊ���ԭ��صķ�Ӧԭ�������������⿼����Ԫ�ؼ��仯�����֪ʶ��������ģʽΪ�߿�����ģʽ���ȵ㣬�����ۺ��Ըߣ���һ�����Ѷȡ�

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ��˵����ȷ����( )
��˵����ȷ����( )