��Ŀ����
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
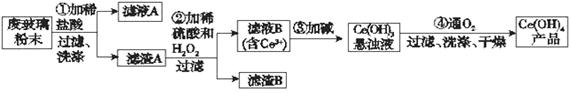
��1�� ��ˮ���εĿ������ã�
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺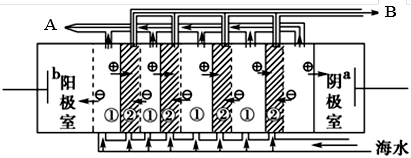
��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�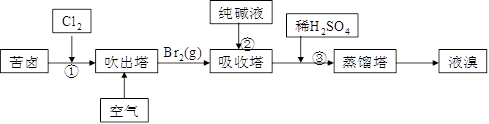
��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
��1�� �� �ᾧ �� ��ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ������ ������������Ҳ�Ʒ֣�
��2���� ��ˮ�к��϶�Mg2+ ��Ca2+�������ӣ����ʱ�����Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �������𰸾��Ʒ֣� ��3�֣� �� ��ˮ
��3����3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
���壬���Br2��Ũ�� �������𰸾��Ʒ֣�
���¶ȹ������Խ�Br2�������������¶ȹ����ֻὫ������ˮ��������������𰸾��Ʒ֣�
���������������1���� �δ���ˮ���о��������أ��õ�Ũ�Ƚϴ��±ˮ���ٽ�±ˮת�Ƶ��ᾧ���нᾧ�����յô��Σ��������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ�����Ӷ��������������������������������������Ͳ�����Ӵ���Ӧ��������ը��ͬʱ�����ϲ�������������Ҳ������������Ӧ�����Ƚϸߣ�
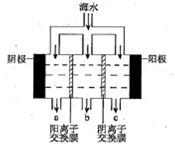
��2���� ��ˮ�и���Ca2+��Mg2+���ӣ�������ˮֱ��ͨ�뵽��װ���У���ˮ�е�Ca2+��Mg2+�����������������ӽ�ϲ���Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �� ͼ�Тڴ���ֱ���糡�������£���Һ�е����Ӿ�������Ǩ�ơ���Ĥֻ����������ͨ�����������ӽ�����������Ĥֻ����������ͨ�����������ӽ������������ʹ��ЩС�ҵ�һ���ֱ�ɺ����Ӻ��ٵĵ�ˮ�ң���ˮ��Ϊ��ˮ�����뵭ˮ�����ڵ�С�����ɾۼ��������ӵ�Ũˮ�ң���ˮ��ΪŨˮ������B����ˮΪ��ˮ��
��3������̼���ƣ����巴Ӧ��BrO3�����ɣ���Ӧ�����ӷ���ʽΪ3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
��Ӣٳ�������Һ����ĺ������ߣ����ֱ������Ʒ�ɱ��ߣ�������Ҫ��һ��Ũ���壬������Ũ�ȣ�
�� �¶ȹ���ˮ�������������к���ˮ�֣��¶ȹ����岻����ȫ���������ʵ͡�
���㣺���麣ˮ���ۺ����á���ˮɹ�Ρ���������������ˮ���Ӻ�ˮ�������ԭ��

��14�֣����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��
��֪����4FeO?Cr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2����
8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3 2NaAlO2+CO2����
2NaAlO2+CO2����
��Cr2CO72-+H2O 2CrO42-+2H+
2CrO42-+2H+
��������ش��������⣺
��1������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4��5��Ӧ��ʹ�� ______________ ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ���� _______________________ ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4���±���������ʵ��ܽ�����ݣ�����III������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7 + 2KCl��K2Cr2O7 ��+ 2NaCl���÷�Ӧ����Һ���ܷ�����������_____________________________��
| ���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
| �ܽ�ȣ�g/100gˮ�� | 0�� | 28 | 35��7 | 4��7 | 163 |
| 40�� | 40��1 | 36��4 | 26��3 | 215 | |
| 80�� | 51��3 | 38 | 73 | 376 | |
��5������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡn g��Ʒ��������� ____ ����д�Լ������ܽ⡢���ˡ��� ����д�Լ������������ա���ȴ���������ø������m g��������Ʒ��������������������Ϊ ���ú�m��n�Ĵ���ʽ��ʾ����
ϡ��Ԫ�������ڱ��Т� B���֡��ƺ���ϵԪ�ص��ܳƣ����Ƕ��Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�������ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ��ҹ��̲��ŷḻ���ƿ�ʯ�� Y2FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�
��֪��I���йؽ��������γ������������ʱ��pH���±���
| | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2.7 | 3.7 |
| Y3+ | 6.0 | 8.2 |
�������ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
��1���ƿ�ʯ��Y2 FeBe2Si2O10������������������ʽ�ɱ�ʾΪ ��
��2������Na2 SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2��������
�����ѡ�����������ѡ���е� �Լ�������ĸ���ţ���
a��NaOH��Һ b����ˮ c��CO2�� d��HNO3
��ͨ�� ��������ʵ�֣���ʵ��������ƣ���
��д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ�� ��
��3��ΪʹFe3+������ȫ�����ð�ˮ����pH =a����aӦ������
�ķ�Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ ����Һ��Fe3+��ȫ�������ж����� ��
��ˮ����Ҫ���ӵĺ������£�
| �ɷ� | ����/(mg/L) | �ɷ� | ����/(mg/L) |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
��1�������£���ˮ��pH��7.5~8.6֮�䣬��ԭ����(�����ӷ���ʽ��ʾ)______________________________��
��2����������������ˮʾ��ͼ��ͼ��ʾ��������(��)���ӽ���Ĥ��������(��)����ͨ���������ϲ�����������������������������ɫ��������ɷ���________��CaCO3������CaCO3�����ӷ���ʽ��_______________��
��3���ú�ˮ��ͬʱ�����Ȼ��ƺͽ���þ��þ�Ļ��������������ͼ��ʾ��
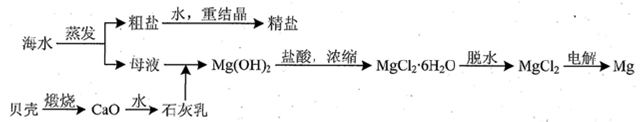
����ʵ�������ɴ��Ρ��ؽᾧ���ƾ��εIJ��������ܽ⡢���ˡ�������ϴ�ӵȲ��裻�й����С������������������ȷ����_____��
a��������Ŀ���ǵõ��ȱ�����Һ
b��������Ŀ������������
c��Ӧ������������Һ
d��Ӧ�������н϶ྦྷ������ʱΪֹ
����MgCl2��Һ�õ�MgCl2?6H2O����ʱ��Ҳ��Ҫ������������Ŀ���ǵõ��ȱ�����Һ���ж���Һ�ѱ��͵�������_________________________��
��4��25��ʱ������Mg(OH)2��Һ��Ũ��Ϊ5��10-4 mol��L��
�ٱ���Mg(OH)2��Һ�еμӷ�̪��������___________________________��
��ijѧϰС��⺣ˮ��Mg2+����(mg/L)�ķ����ǣ�ȡһ������ĺ�ˮ����������_________���ټ�������NaOH����Mg2+תΪMg(OH)2��25�棬�÷�����õ�Mg2+���������1272mg/L�ġ���ֵ���ȣ�������ԼΪ______��[����2λС������ˮ�б���Mg(OH)2��Һ���ܶȶ���l g/cm3��]��
�Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȡ�ʵ����ģ�ҵ���������Ʊ�����(Fe2O3)���������£�
��1���������ijɷ�������������������� �� д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ�� ��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ��_________��������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
��3������A����Ҫ�ɷ�Ϊ ����ҺB���Ի��յ�������______________________��
��4������ϴ�ӹ��̵�ʵ����� ��
��5����֪����������Ϊw kg�����������Ʊ������У���Ԫ�����25%�����յõ����������Ϊm kg����ԭ������������Ԫ����������Ϊ ��������������ʽ��ʾ����
����֪���ԭ��������O 16 S 32 Fe 56 ��