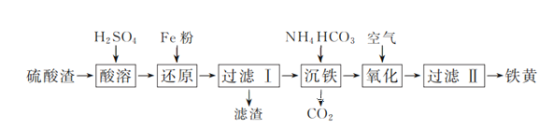
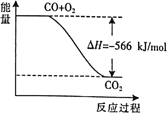
��Ŀ����
����Ŀ����ͭ����������ʹ�õĺϽ�֮һ����Ҫ��п��ͭ��ɡ��ش��������⣺
��1��ͭԭ�Ӻ�����ӹ���__�ֲ�ͬ�˶�״̬����̬ͭԭ�ӵĺ���ռ������ܲ���ӵĵ���������ͼ��״Ϊ__��
��2������ͭ��пԭ�ӽṹ��֪�ڶ�������I2(Zn)__I2(Cu)��������������С���� ��
��3������ɫ����ͭ��Һ[Cu(H2O)4]2+�м����Թ����İ�ˮ����Һ��Ϊ����ɫ[Cu(NH3)4]2+��
��H2O��������ԭ�ӵ��ӻ�����Ϊ__�������еļ��ǣ�H2O__NH3(��������������С����)��
��ͨ������ʵ�������֪����Cu2+����λ������H2O__NH3(��������������С����)��
�۰�����(BH3��NH3)������Ϊ������ʹ�õı�ѡ����Դ���ⴢ��IJ��ϡ�
�����백�����ǵȵ��������__(����)��
A.���� B.H2O2 C.H3PO4 D.S8
��д��BH3��NH3�Ľṹʽ �ṹ��������λ��������ʾ __��
��4��ij����ͭ�����ṹ��ͼ��ʾ��
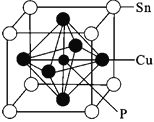
���仯ѧʽΪ__��
���������ܶ�Ϊ8.82g/cm3�������Cuԭ�Ӻ˼��Ϊ__cm(��NA��ʾ�����ӵ���������M��ʾ�þ����Ħ������)��
���𰸡�29 ���� С�� sp3 С�� С�� A 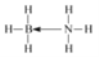 SnCu3P
SnCu3P 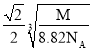
��������
(1)Cuλ�����ڱ��е�4���ڵڢ�B�壬��̬��������Ų�Ϊ[Ar]3d104s1��
(2)����Zn��Cu�ĵ����Ų�ʽ���ǵ����ܴ�С��
(3)�ٸ���VSEPR���ۺ��ӻ���������жϣ��µ��Ӷ�����ϵ��Ӷ�֮��ij������ڼ��ϵ��Ӷ�����ϵ��Ӷ�֮��ij�����
������������ɸ��ȶ��������ת����
��ԭ����Ŀ��ȡ��۵���������ȵ�����Ϊ�ȵ����壻
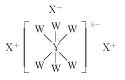
��BH3-NH3��B���ڿչ����N���ڹµ��Ӷԣ���N��B֮���γ���λ����
(4)�پ�����Pԭ��λ�����ģ�����һ����ԭ�ӣ�������ÿ�����ĺ�һ��Cu��ÿ��Cu��̯��һ��������ռ![]() �������嶥��Sn��̯��ÿ��������ԭ��Ϊ
�������嶥��Sn��̯��ÿ��������ԭ��Ϊ![]() ���ݴ˵õ���ѧʽ��
���ݴ˵õ���ѧʽ��
����������߳�Ϊx��ͭԭ�Ӽ��������Ϊa����a2=(![]() )2+(
)2+(![]() )2��a=
)2��a=![]() x������ܶ���=
x������ܶ���=![]() ��V=x3��1����������m=
��V=x3��1����������m=![]() g������x�õ�ͭԭ�Ӽ�������롣
g������x�õ�ͭԭ�Ӽ�������롣
(1)Cuλ�����ڱ��е�4���ڵڢ�B�壬Ϊ29��Ԫ�أ���ͭԭ�Ӻ�����ӹ���29�ֲ�ͬ�˶�״̬����̬��������Ų�Ϊ[Ar]3d104s1�����̬ͭԭ�ӵĺ���ռ������ܲ���ӵĵ���������ͼ��״Ϊ���Σ�
(2)п�ĵڶ�������I2(Zn)С��ͭ�ĵڶ�������I2(Cu)��Zn�ļ۵����Ų�ʽΪ3d104s2��Cu�ļ۵����Ų�ʽΪ3d104s1��Cuʧȥһ�������ڲ�ﵽ���ͣ���ʧȥһ�����ӱȽ����ѣ�Znʧȥһ�����Ӽ۲��Ϊ3d104s1����ʧȥһ�����ӱ�Cu+���ף����Եڶ����������С��
(3)�ٶ���H2O������VSEPR���ۣ�VP=BP+LP=2+![]() =4��������OΪsp3�ӻ���
=4��������OΪsp3�ӻ���
�µ��Ӷ�����ϵ��Ӷ�֮��ij������ڼ��ϵ��Ӷ�����ϵ��Ӷ�֮��ij�����H2O�ŵ��Ӷ���Ŀ����NH3�ŵ��Ӷ���Ŀ��������еļ��ǣ�H2OС��NH3��
������������ɸ��ȶ��������ת���������ж�NH3��H2O��Cu2+����λ������NH3��H2O��
��Bԭ���пչ����NH3��Nԭ����1�Թµ��Ӷԣ�Nԭ���ṩ�µ��ӶԸ�Bԭ���γ���λ�����백���黥Ϊ�ȵ�����ķ��ӣ�������2��Cԭ�Ӵ���B��Nԭ�ӣ��백����ȵ�����һ�ַ���Ϊ��C2H6���ʴ�ΪA��
��BH3-NH3��B���ڿչ����N���ڹµ��Ӷԣ���N��B֮���γ���λ����������ṹʽΪ�� ��
��
(4)�پ�����Pԭ��λ�����ģ�����һ����ԭ�ӣ�������ÿ�����ĺ�һ��Cu��ÿ��Cu��̯��һ��������ռ![]() �������嶥��Sn��̯��ÿ��������ԭ��Ϊ
�������嶥��Sn��̯��ÿ��������ԭ��Ϊ![]() ���ݴ˵õ���ѧʽSnCu3P��
���ݴ˵õ���ѧʽSnCu3P��
��Ħ������Ϊ342g/mol����һ����������m=![]() g����������߳�Ϊx��ͭԭ�Ӽ��������Ϊa����a2=(
g����������߳�Ϊx��ͭԭ�Ӽ��������Ϊa����a2=(![]() )2+(
)2+(![]() )2��a=
)2��a=![]() x������ܶ���=
x������ܶ���=![]() ��V=x3=
��V=x3=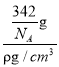 =
=![]() cm3��x=
cm3��x=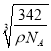 cm��a=
cm��a=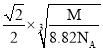 cm��
cm��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�