��Ŀ����
����Ҫ��ش����и���
�������� ���� ��NH3������������Һ ��Һ̬�Ȼ��� ��AgCl����ޱ����� �����ǣ���ջش�����ţ���
���������У�1�����ڵ���ʵ��� ����2�����ڷǵ���ʵ��� ��
��3������ǿ����ʵ��� ����4���ܵ������ ��
����д�����з�Ӧ�Ļ�ѧ����ʽ��
����NaHCO3���ɵĻ��Ϸ�Ӧ
�� ��MgCl2�μӵķֽⷴӦ
�� ��Fe2O3�μӵ��û���Ӧ
�� ��HNO3���ɵĸ��ֽⷴӦ
(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2������֮���� ��ͬ������CH4��SO2�����֮���� ������������ԭ�Ӹ���������ȣ���CH4��SO2������֮���� ��
����������Ƭ��ʯī��������a��b���ַ�ʽ����ʢ��ϡ��������Һ�ͷ�̪��Һ�����Һ�IJ��������У�����һ��ʱ������ȹ۲쵽��Һ���������� ������ţ���
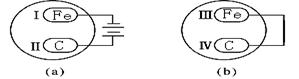
�������� ���� ��NH3������������Һ ��Һ̬�Ȼ��� ��AgCl����ޱ����� �����ǣ���ջش�����ţ���
���������У�1�����ڵ���ʵ��� ����2�����ڷǵ���ʵ��� ��
��3������ǿ����ʵ��� ����4���ܵ������ ��
����д�����з�Ӧ�Ļ�ѧ����ʽ��
����NaHCO3���ɵĻ��Ϸ�Ӧ
�� ��MgCl2�μӵķֽⷴӦ
�� ��Fe2O3�μӵ��û���Ӧ
�� ��HNO3���ɵĸ��ֽⷴӦ
(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2������֮���� ��ͬ������CH4��SO2�����֮���� ������������ԭ�Ӹ���������ȣ���CH4��SO2������֮���� ��
����������Ƭ��ʯī��������a��b���ַ�ʽ����ʢ��ϡ��������Һ�ͷ�̪��Һ�����Һ�IJ��������У�����һ��ʱ������ȹ۲쵽��Һ���������� ������ţ���
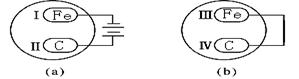
| A����͢� | B����͢����� | C����͢� | D����͢����� |
����12�֣�ÿ��1�֣�����1���ܢݢ��� 2���ڢ���3���ܢ���4���٢�����2NaOH+CO2+H2O=2NaHCO3��NaOH+CO2=NaHCO3 �� MgCl2 Mg+Cl2���� Fe2O3+2Al
Mg+Cl2���� Fe2O3+2Al 2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
 Mg+Cl2���� Fe2O3+2Al
Mg+Cl2���� Fe2O3+2Al 2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B���������������ʺͷǵ���ʶ��ǻ���������ǰ���½��з��������Ϊ��1���ܢݢޣ� 2���ڢ� ��3���ܢ� ��4���٢� ��������ϵƽʱ��ѧ�Ļ���֪ʶ����ɻ�ѧ����ʽ�����ʾ��(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2�����ʵ�����ͬ����������֮����Ħ������֮�ȣ���Ϊ1��4 ��ͬ������CH4��SO2�����֮������Ħ�������ķ��ȡ�����������ԭ�Ӹ���������ȣ����趼Ϊ1molת��ΪCH4��SO2������֮����3��20 ��������a�ǵ��أ�bΪԭ��أ���a���൱�ڵ��ˮ���������������ӷŵ磬��Χ�ʼ��ԣ�������������ʴ����̼����Χ��������������������ȹ۲쵽��Һ���������Ǣ�͢�������

��ϰ��ϵ�д�
�����Ŀ