��Ŀ����
����Ŀ����ѧ��Ӧԭ���ڿ��к�ũҵ�������й㷺Ӧ�á�
��1��ij��ѧ��ȤС����й�ҵ�ϳɰ���ģ���о�����Ӧ�ķ���ʽΪN2��g��+3H2��g��![]() 2NH3��g�� ��H<0����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��������������c��N2����ʱ����t���ı仯����ͼ��ʾ��
2NH3��g�� ��H<0����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��������������c��N2����ʱ����t���ı仯����ͼ��ʾ��

ʵ�����ӳ�ʼ��ƽ��Ĺ����У��÷�Ӧ��ƽ����Ӧ����v��NH3��=_______________����ʵ������ȣ�ʵ������ʵ�������ı��ʵ�������ֱ�Ϊ����ѡ���е�______________��_____________������ĸ�������
A����ѹǿ B��Сѹǿ C�����¶� D�����¶� Eʹ�ô���
��2����֪NO2��N2O4�����ת����2NO2��g��![]() N2O4��g����
N2O4��g����
T��ʱ����0.40 mol NO2��������ݻ�Ϊ2L���ܱ������У��ﵽƽ����������c��N2O4��=0.05 mol/L����÷�Ӧ��ƽ�ⳣ��K=_______________��
���𰸡���1�� 0.008molL-1min-1 E b ��2��5Lmol-1
��������
�����������1���������֪������10minʱ�ﵽƽ�⣬��ʱ������Ũ�ȱ仯Ϊ0.04mol/L�����ݷ���ʽ��֪��������Ũ�ȱ仯��0.08mol/L������v=��c/��t��֪v��NH3��=0.08mol/L��10min=0.008molL-1min-1������ͼ���֪������ƽ���ʱ������̣�����ƽ��ʱN2��Ũ��������ͬ����ѧƽ�ⲻ�ƶ�������������ȼ��˴�������ѡE���������ȽϿ�֪���������ʱ���С��ƽ��ʱ������Ũ�ȸߣ���ƽ�������ƶ�����������С��ѹǿ����ѡb��
��2����2NO2��g��![]() N2O4��g��
N2O4��g��
��ʼʱ 0.2molL-1 0
ת�� 0.1molL-1 0.05molL-1
ƽ��ʱ 0.1molL-1 0.05molL-1
��ƽ�ⳣ��K=![]() Lmol-1=5Lmol-1��
Lmol-1=5Lmol-1��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
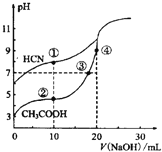
ȫ�̽��ϵ�д�����Ŀ����50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
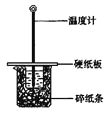
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ ������ƫ��ƫС����Ӱ������
��3�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� ��������ȡ�����������������к��� ��������ȡ��������������������
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� ������ƫ��������ƫС��������Ӱ��������ͬ�� ����KOH����NaOH���ⶨ����� __ __
��5�����Ǽ�¼��ʵ���������£�
ʵ �� �� Ʒ | �� Һ �� �� | �к�����H | |||
t1 | t2 | ||||
�� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� | |
�� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� | |
��֪��Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
����������ϱ���
������ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ�� ��