��Ŀ����
ijͬѧ�����һ���״�ȼ�ϵ�أ����øõ�ص��200mL����Ũ��NaCl��CuSO4�����Һ����װ����ͼ��
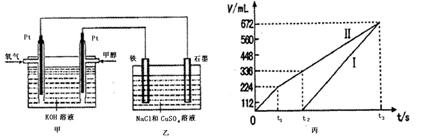
��1��д������ͨ��״���һ���ĵ缫��Ӧʽ ��
��2������������������������������ʱ��仯�Ĺ�ϵ���ͼ��ʾ����������ѻ���ɱ�״���µ��������д����t1��,ʯī�缫�ϵĵ缫��Ӧʽ ��ԭ�����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��CuSO4�����ʵ���Ũ��Ϊ mol/L����������Һ������䣩
��3�������t3ʱ������ˮ������Ϊ g��

��1��д������ͨ��״���һ���ĵ缫��Ӧʽ ��
��2������������������������������ʱ��仯�Ĺ�ϵ���ͼ��ʾ����������ѻ���ɱ�״���µ��������д����t1��,ʯī�缫�ϵĵ缫��Ӧʽ ��ԭ�����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��CuSO4�����ʵ���Ũ��Ϊ mol/L����������Һ������䣩
��3�������t3ʱ������ˮ������Ϊ g��
��1��CH3OH-6e��+8OH��=CO32��+6H2O��2�֣�
��2��4OH--4e��=O2��+2H2O ��2�֣� 0.1��2�֣� 0.1��2�֣�
(3)0.72 ��2�֣�
��2��4OH--4e��=O2��+2H2O ��2�֣� 0.1��2�֣� 0.1��2�֣�
(3)0.72 ��2�֣�
��1����ͼΪ�״�ȼ�ϵ�أ� 2CH3OH+3O2=2CO2+4H2O ��
CO2+2KOH=K2CO3+H2O ��
��+�ڡ�2���ã��״�ȼ�ϵ�ص��ܻ�ѧ��ӦΪ��2CH3OH+3O2+4KOH=6H2O+2K2CO3
�����ӷ�ӦΪ��2CH3OH+3O2+4OH-=6H2O+2CO32-
��+����O2+2H2O+4e��=4OH��
��-��=�����ӷ�Ӧ-��+����3���ã�2CH3OH+16OH��-12e��=12H2O+2CO32-
���ã�CH3OH - 6e��+ 8OH�� = CO32��+ 6H2O
��2����ͼΪ���أ���Ƭ�Ӽ�ͼ�ĸ���������Ƭ�����ص�������
ʯī���Ӽ�ͼ����������ʯī�������ص�������
��Һ�������ӣ�Cl-��OH-��SO42-����������,�ҷŵ�˳��Ϊ��Cl-��OH-��SO42-��
�缫��ӦΪ����2Cl--2e-=Cl2������4OH--4e��=O2��+2H2O
�����ӣ�Cu2+��H+��Na+����������,�ҷŵ�˳��Ϊ��Cu2+��H+��Na+��
�缫��ӦΪ����Cu2++2e-=Cu����2H++2e-=H2��
�ʱ�ͼ�У�I�߶�Ӧ��������Ƭ���ķ�Ӧ��II�߶�Ӧ������ʯī�����ķ�Ӧ��
������ͼ����t1ʱ������V��Cl2��=224ml����״����n(Cl2)="0.01" mol��
2Cl--2e-=Cl2��
2 1
n(Cl-) 0.01mol����n(Cl-)= 0.02mol��C��NaCl��="0.02mol/0.2L=0.1" mol/L��
V��O2��="672ml-224ml=448" ml����״����n(O2)="0.02" mol��������ʧ���ӵ����ʵ���Ϊ��
2Cl-- 2e- = Cl2�� 4OH-- 4e�� = O2��+2H2O
2 1 4 1
0.02mol 0.01mol 0.08mol 0.02mol
��������ת���غ㣨��Ϊ0.02mol+ 0.08mol=0.1mol�����������õ��ӵ����ʵ���Ϊ��
V��H2��="672ml," ��״����n(H2)=0.03mol��
2H++ 2e- = H2�� �� Cu2+ + 2e- =Cu
2 1 1 2
0.06mol 0.03mol 0.02mol 0.04mol
C��CuSO4��="0.02mol/0.2L=0.1" mol/L��
��3��2H2O=2H2��+O2��
36g 1mol
0.72g 0.02mol
CO2+2KOH=K2CO3+H2O ��
��+�ڡ�2���ã��״�ȼ�ϵ�ص��ܻ�ѧ��ӦΪ��2CH3OH+3O2+4KOH=6H2O+2K2CO3
�����ӷ�ӦΪ��2CH3OH+3O2+4OH-=6H2O+2CO32-
��+����O2+2H2O+4e��=4OH��
��-��=�����ӷ�Ӧ-��+����3���ã�2CH3OH+16OH��-12e��=12H2O+2CO32-
���ã�CH3OH - 6e��+ 8OH�� = CO32��+ 6H2O
��2����ͼΪ���أ���Ƭ�Ӽ�ͼ�ĸ���������Ƭ�����ص�������
ʯī���Ӽ�ͼ����������ʯī�������ص�������
��Һ�������ӣ�Cl-��OH-��SO42-����������,�ҷŵ�˳��Ϊ��Cl-��OH-��SO42-��
�缫��ӦΪ����2Cl--2e-=Cl2������4OH--4e��=O2��+2H2O
�����ӣ�Cu2+��H+��Na+����������,�ҷŵ�˳��Ϊ��Cu2+��H+��Na+��
�缫��ӦΪ����Cu2++2e-=Cu����2H++2e-=H2��
�ʱ�ͼ�У�I�߶�Ӧ��������Ƭ���ķ�Ӧ��II�߶�Ӧ������ʯī�����ķ�Ӧ��
������ͼ����t1ʱ������V��Cl2��=224ml����״����n(Cl2)="0.01" mol��
2Cl--2e-=Cl2��
2 1
n(Cl-) 0.01mol����n(Cl-)= 0.02mol��C��NaCl��="0.02mol/0.2L=0.1" mol/L��
V��O2��="672ml-224ml=448" ml����״����n(O2)="0.02" mol��������ʧ���ӵ����ʵ���Ϊ��
2Cl-- 2e- = Cl2�� 4OH-- 4e�� = O2��+2H2O
2 1 4 1
0.02mol 0.01mol 0.08mol 0.02mol
��������ת���غ㣨��Ϊ0.02mol+ 0.08mol=0.1mol�����������õ��ӵ����ʵ���Ϊ��
V��H2��="672ml," ��״����n(H2)=0.03mol��
2H++ 2e- = H2�� �� Cu2+ + 2e- =Cu
2 1 1 2
0.06mol 0.03mol 0.02mol 0.04mol
C��CuSO4��="0.02mol/0.2L=0.1" mol/L��
��3��2H2O=2H2��+O2��
36g 1mol
0.72g 0.02mol

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
 NiOOH + MH������������ȷ���ǣ� ��
NiOOH + MH������������ȷ���ǣ� ��