��Ŀ����
ij��ɫ��Һ���ܺ������������еļ��֣�
(1)����ʹƷ����Һ��ɫ��˵���������в���_____(�ѧʽ)��
(2)����ɫ��Һ�����ٴ����ļ������Σ���д��ȫ�����ܵ����(��д��Ӧ����ĸ)��
��һ�������__________________________________��
�ڶ��������__________________________________��
�����������__________________________________��
�����������__________________________________��
(�ɲ�������Ҳ�ɲ���)
| A���Ȼ��ƣ� | B�����ƣ� | C���������ƣ� | D����������ƣ�(E)�����ƣ�(F)̼���ơ������Һ�м�������ϡ���ᣬ��dz��ɫ�ij���������ͬʱ������������������г�������ζ����ʹ����ʯ��ˮ����ǣ�����ʹƷ����Һ��ɫ����������ʵ������ش��������⣺ |
(2)����ɫ��Һ�����ٴ����ļ������Σ���д��ȫ�����ܵ����(��д��Ӧ����ĸ)��
��һ�������__________________________________��
�ڶ��������__________________________________��
�����������__________________________________��
�����������__________________________________��
(�ɲ�������Ҳ�ɲ���)
(1)SO2 (2)B��C��F B��D��F
����dz��ɫ�������������ܵķ�Ӧԭ��Ϊ2Na2S+Na2SO3+3H2SO4=3S��+3Na2SO4+3H2O��Na2S2O3+H2SO4=S��+SO2��+H2O+Na2SO4���������г�������ζ����˵��������ΪH2S���ʱغ�Na2S������ʹʯ��ˮ���ǣ�����ʹƷ����ɫ����˵�������岻��SO2��һ����CO2���ʱغ�Na2CO3��
���ϣ��غ�������ΪNa2S��Na2CO3���ټ���һ���ܲ���dz��ɫ���������ʣ���Na2SO3��Na2S2O3���������ٴ������������
���ϣ��غ�������ΪNa2S��Na2CO3���ټ���һ���ܲ���dz��ɫ���������ʣ���Na2SO3��Na2S2O3���������ٴ������������

��ϰ��ϵ�д�
�����Ŀ
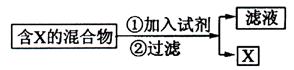
 ��1��
��1�� ����Ӧ����������______________����������Ϊ__________
����Ӧ����������______________����������Ϊ__________ ���½���
���½��� ��
��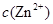 �������ӣ������趨ʱ��ȥ���е�
�������ӣ������趨ʱ��ȥ���е�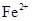 ��
�� ���±�Ϊ�������ʵ��ܶȻ���
���±�Ϊ�������ʵ��ܶȻ���
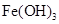








 ����
����

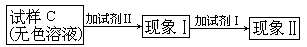
 ��Һ+���� ��B+C
��Һ+���� ��B+C