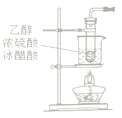
��Ŀ����
����Ŀ�������Ȼ�ѧ����ʽ��������ȷ����
A. ��֪HCl��NaOH��Ӧ���к�����H��-57.3 kJ��mol��1����CH3COOH(aq)��NaOH(aq)=CH3COONa(aq)��H2O(l) ��H��-57.3kJ��mol��1
B. 500�桢30 MPa�£�N2(g)��3H2(g)![]() 2NH3(g) ��H��-92.4 kJ��mol��1��0.5 mol N2(g)��1.5 mol H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)����46.2 kJ
2NH3(g) ��H��-92.4 kJ��mol��1��0.5 mol N2(g)��1.5 mol H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)����46.2 kJ
C. ��֪������ȼ����Ϊ286 kJ��mol��1����2H2O(g)===2H2(g)��O2(g)����H����572 kJ��mol��1
D. �����ȼ����Ϊ890.3 kJ��mol��1�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)��CO2(g)��2H2O(l)����H����890.3 kJ��mol��1
���𰸡�D
��������
A.����������Ҫ���ղ���������
B.���������������ȷ�Ӧǰ�ɱ�����С�������൱�ڼ�ѹ������ƽ�������ƶ���
C. ȼ����ָ�����ȶ������ˮΪҺ̬��
D. ȼ����ָ�����ȶ������ˮΪҺ̬��������̼Ϊ��̬��
A. CH3COOH��������������������������CH3COOH(aq)��NaOH(aq)=CH3COONa(aq)��H2O(l) ��H>-57.3kJ��mol��1��A������
B. ��Ӧ����1 mol N2(g)��3 mol H2������92.4 kJ���ֽ�0.5 mol N2(g)��1.5 mol H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)�Ĺ�������Ч�ڼ�ѹ�Ĺ�����ƽ���������ų�������С��46.2 kJ��B������
C. ������ȼ����ָ����1mol������ȫȼ������Һ̬ˮ����286 kJ��mol��1������2H2O(l)===2H2(g)��O2(g)����H����572 kJ��mol��1����2H2O(g)===2H2(g)��O2(g)����H<��572 kJ��mol��1��C������
D. �����ȼ����ָ����1mol����������ȫȼ������Һ̬ˮ����Ϊ890.3 kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)��CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��D��ȷ��
��������������ѡD��

����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g��![]() CO��g����H2O��g����
CO��g����H2O��g����
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK =__________________��
��2���÷�ӦΪ__________��Ӧ�������ȡ����ȡ�����
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������_______________��
a��������ѹǿ���� b����������� c��CO������
c��������H2�������棨H2O�� d��c��CO2����c��CO��
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c��CO2����c��H2����c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ________����