��Ŀ����
�Ȼ���ͭ��CuCl���㷺���ڻ�����ӡȾ���л��ϳɵ���ҵ��CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ���ڳ�ʪ��������ˮ���������Ժ���ͭ����Ҫ�ɷ���Cu������CuO��Ϊԭ�ϣ���������������ֽ⼼�������Ȼ���ͭ�Ĺ��չ�������ͼ��ʾ��

�ش��������⣺
��1��CuCl��CuԪ�������ڱ��е�λ��Ϊ___________��
��2���������NԪ�ر���ԭΪ��ͼۣ���Cu�ܽ�����ӷ���ʽΪ________���ܽ��¶�Ӧ������60~70�棬ԭ����______________��
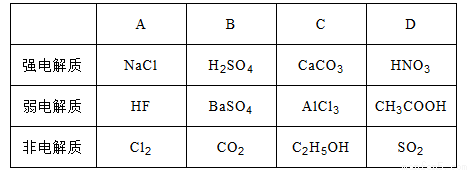
��3��д�����������Ҫ��Ӧ�����ӷ���ʽ________��(NH4)2SO3Ҫ�ʵ�������Ŀ���У���֤Cu2+�Ļ�ԭ���ʣ�__________����֪NH4Cl��Cu2+�����ʵ���֮�� ��Cu2+�����ʵĹ�ϵ��ͼ��ʾ�����Ȼ���������ӵ�һ��Ũ�Ⱥ��Ȼ���ͭ�ij����ʼ��٣�ԭ����________��
��Cu2+�����ʵĹ�ϵ��ͼ��ʾ�����Ȼ���������ӵ�һ��Ũ�Ⱥ��Ȼ���ͭ�ij����ʼ��٣�ԭ����________��
��4����������Ҵ�ϴ�ӵ�Ŀ����__________��
��5���Ȼ���ͭ�Ķ���������
�ٳ�ȡ��Ʒ0.250g��10mL������FeCl3��Һ��250mL��ƿ�У�����ܽ⣻
����0.100mol��L��1������[Ce(SO4)2]����Һ�ⶨ����֪��CuCl+FeCl3=CuCl2+FeCl2��Fe2++Ce4+=Fe3++Ce3+��
����ƽ��ʵ�������±���ƽ��ʵ�������ܳ���1%����
ƽ��ʵ����� | 1 | 2 | 3 |
0.250g��Ʒ�������������Һ�������mL�� | 24.35 | 24.05 | 23.95 |
����Ʒ��CuCl�Ĵ���Ϊ_______���������3λ��Ч���֣���
����ͼ��ʾװ�ý�������ʵ�飬a��b��c����װ�Լ����±���ʾ������ʵ����������۶�Ӧ��ϵ��ȷ��һ����

ѡ�� | a | b | c | ���� | ���� |
A | ����ʳ��ˮ | ̼���� | ��ˮ | c����Һ��ɫ��ȥ | ��Ȳ������ԭ��Ӧ |
B | Ũ���� | KMnO4���� | NaBr��Һ | c����Һ����ɫ���ɫ | Cl2�������Ա�Br2ǿ |
C | ϡ���� | ����ʯ | Na2SiO3��Һ | c���а�ɫ��״�������� | ̼������Աȹ���ǿ |
D | ���� | Na2SO3���� | ����KMnO4��Һ | c����Һ��ɫ��ȥ | SO2����Ư���� |
A. A B. B C. C D. D
��ҵ����ú��ˮΪԭ��ͨ��һϵ��ת���ɱ�Ϊ�����Դ������ҵԭ�ϼ״���
��1����֪��C(s)+O2(g)=CO2(g) ��H1
��2H2(g)+O2(g)=2H2O (l) ��H2
��H2O (l)= H2O (g) ��H3
��̼��ˮ������ӦC(s)+2H2O(g) CO2(g)+2H2(g)�Ħ�H =________��
CO2(g)+2H2(g)�Ħ�H =________��
��2����ҵ��Ҳ���Խ�����������Ӧ�õ���CO2��H2��һ���ϳɼ״�����Ӧ����ʽΪ��CO2(g)��3H2(g) CH3OH(g)��H2O(g����H��0
CH3OH(g)��H2O(g����H��0
�ٹ�ҵ����������CO2��H2��ת����________���ǰ�ߴ������ߴ���һ�������жϡ�����Ϊ����״��IJ��ʿ��Բ�ȡ�Ĵ�ʩ��_______________�������㣩��
����һ���º����ܱ������г���1 mol CO2��3 mol H2����������Ӧ�����CO2��CH3OH(g)Ũ����ʱ��仯����ͼ��ʾ�����¶��µ�ƽ�ⳣ��Ϊ______��������λ��Ч���֣���
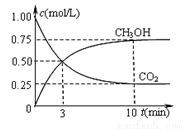
�ı��¶ȣ�ʹ��ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2��mol�� | H2��mol�� | CH3OH��mol�� | H2O��mol�� | |
��Ӧ�� ���º��� | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ�� ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�ٴﵽƽ��ʱ����Ӧ��Աȣ�ƽ�ⳣ��K(��)___K(��)�����������������=������ͬ����ƽ��ʱCH3OH��Ũ��c(��)___c(��)��
�ڶԷ�Ӧ��ǰ10 min�ڵ�ƽ����Ӧ���ʦ�(CH3OH)=______����30 minʱֻ���������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��_____�ƶ��������������������