��Ŀ����
��1�����Թ������һС��ͭƬ��ע��һ�������Ũ���ᣬ���Թܼ��ȣ�ʹ֮��Ӧ����ͭƬ����ʣ�������Ƿ��������?
�ٽ��ۼ������ǣ�_________________________________________��
����100����18 mol/L��H2SO4��Һ�м���������ͭƬ�����ȣ���ַ�Ӧ����ԭ��H2SO4�����ʵ���
A��С��0��9 mol B������0��9 mol C����0��9 mol��1.8 mol֮�� D������1��8 mol
��50g�ĵ������ȵ�100��ʧȥ���ֽᾧˮ��������Ϊ35.6g����ʧȥˮ�������ͭ����Ļ�ѧʽ��
A��CuSO4��H2O B��CuSO4��2H2O C��CuSO4��3H2O D��CuSO4
��2��������пͶ�뵽һ������Ũ�����У���ַ�Ӧ���ռ���SO2��H222.4L(��״��)��
�ټ����ܲ���H2��ԭ����____________ __________________
�������仯������Ũ����ǿ�����Եķ�Ӧ����ʽ�ǣ�
�۷�Ӧ�����Ľ���п������Ϊ_________g��
�����������ܷ��������ĵ���������ʵ����� _ (��ܡ����ܡ�)
�ٽ��ۼ������ǣ�_________________________________________��
����100����18 mol/L��H2SO4��Һ�м���������ͭƬ�����ȣ���ַ�Ӧ����ԭ��H2SO4�����ʵ���
A��С��0��9 mol B������0��9 mol C����0��9 mol��1.8 mol֮�� D������1��8 mol
��50g�ĵ������ȵ�100��ʧȥ���ֽᾧˮ��������Ϊ35.6g����ʧȥˮ�������ͭ����Ļ�ѧʽ��
A��CuSO4��H2O B��CuSO4��2H2O C��CuSO4��3H2O D��CuSO4
��2��������пͶ�뵽һ������Ũ�����У���ַ�Ӧ���ռ���SO2��H222.4L(��״��)��
�ټ����ܲ���H2��ԭ����____________ __________________
�������仯������Ũ����ǿ�����Եķ�Ӧ����ʽ�ǣ�
�۷�Ӧ�����Ľ���п������Ϊ_________g��
�����������ܷ��������ĵ���������ʵ����� _ (��ܡ����ܡ�)
(12��)��1���ٷ��淴Ӧ���У�����Ũ�Ȳ��Ͻ��ͣ�ϡ������ͭ����Ӧ�������������ʣ�� ��A ��A
(2)���淴Ӧ�IJ��Ͻ��У�Ũ����Ũ�Ȳ��Ͻ��ͣ���пϡ���ᷴӦ����������
��Zn+2H2SO4(Ũ)��ZnSO4+SO2��+2H2O ��65 �ܲ���
(2)���淴Ӧ�IJ��Ͻ��У�Ũ����Ũ�Ȳ��Ͻ��ͣ���пϡ���ᷴӦ����������
��Zn+2H2SO4(Ũ)��ZnSO4+SO2��+2H2O ��65 �ܲ���
�����������1���������淴Ӧ���У�����Ũ�Ȳ��Ͻ��ͣ�ϡ������ͭ����Ӧ�������������ʣ�ࡣ
��Ũ��������ʵ�����1.8mol�����Ը��ݷ�ӦʽCu��2H2SO4(Ũ)
 CuSO4��2H2O��SO2��������Ϣٿ�֪��ʵ�ʱ���ԭ��������ʵ���С��0.9mol����ѡA��
CuSO4��2H2O��SO2��������Ϣٿ�֪��ʵ�ʱ���ԭ��������ʵ���С��0.9mol����ѡA����50g�ĵ������ȵ�100��ʧȥ���ֽᾧˮ��������Ϊ35.6g����ʧȥ��ˮ��������50g��35.6g��14.4g�����ʵ�����0.8mol�����������ʵ�����0.2mol�����ƺ���1molˮ����˵����Ӧ���е�ˮ�����ʵ���ˮ0.2mol�����Ի�����Ļ�ѧʽ��CuSO4��H2O����ѡA��
��2���������淴Ӧ�IJ��Ͻ��У�Ũ����Ũ�Ȳ��Ͻ��ͣ���пϡ���ᷴӦ������������
������Ũ����ǿ�����Եķ�Ӧ����ʽ��Zn+2H2SO4(Ũ)��ZnSO4+SO2��+2H2O��
�ۻ���������ʵ�����1mol�����ڲ���������������SO2���ڷ�Ӧ�ж���ת��2�����ӵģ����Թ��Ƶ��ӵ�ʧ�غ��֪���μӷ�Ӧ��п�����ʵ���Ҳ��1mol��������65g��
�����ڲ���ȷ��SO2�����ʵ�����������ȷ�����ĵ���������ʵ�����
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���������С�������ע�ضԻ�����֪ʶ������ѵ����ͬʱ����Ҫ�Dz�������ѧ���Ľ��ⷽ���뼼�ɣ������ڵ���ѧ����ѧϰ��Ȥ������ѧ������֪��������Ĺؼ�������Ũ����Ļ�ѧ���ʣ��Լ����ú��غ㷨�ڻ�ѧ�����е���ҪӦ�á�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
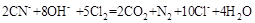 �������жϴ������
�������жϴ������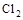
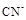 ��
��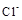
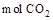 ����ʱ����Һ�������ӵ����ʵ�������1
����ʱ����Һ�������ӵ����ʵ�������1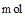
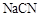 ��ˮ��Һ�ʼ��ԣ�˵��
��ˮ��Һ�ʼ��ԣ�˵��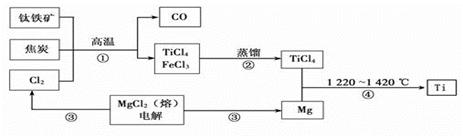
 2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�